9 Regulation of Gene Expression
Learning Objectives
After exploring this chapter, you should be able to
- Describe mechanisms that regulate gene expression in eukaryotes, including promoter activity and chromatin structure.
- Explain when splicing occurs and how it contributes to mature mRNA formation.
- Define gene networks and explain how gene interactions regulate biological functions.
- Explain how gene networks can coordinate the expression of multiple genes to affect traits and behaviors.
- Analyze how alternative splicing can produce different protein isoforms from a single gene.
- Explain how environmental signals influence gene expression.
- Create a model showing the interaction between multiple genes in a regulatory network.
Gene expression is the process of turning on a gene to produce RNA and protein. For a cell to function properly, it must produce the right proteins at the right time and in the right amounts. Both unicellular and multicellular organisms control which genes are expressed, when they are expressed, and how much protein is made.
In multicellular organisms such as ourselves, gene regulation allows for specialization among different cell types. A muscle cell and a skin cell contain a copy of the same chromosomes and genes, but they function differently because they express different subsets of those genes. Likewise, unicellular organisms adjust gene expression in response to environmental signals, such as nutrients or stress. By controlling which gene are expressed and when allows cells to use energy and resources more efficiently.
Prokaryotic versus Eukaryotic Gene Expression
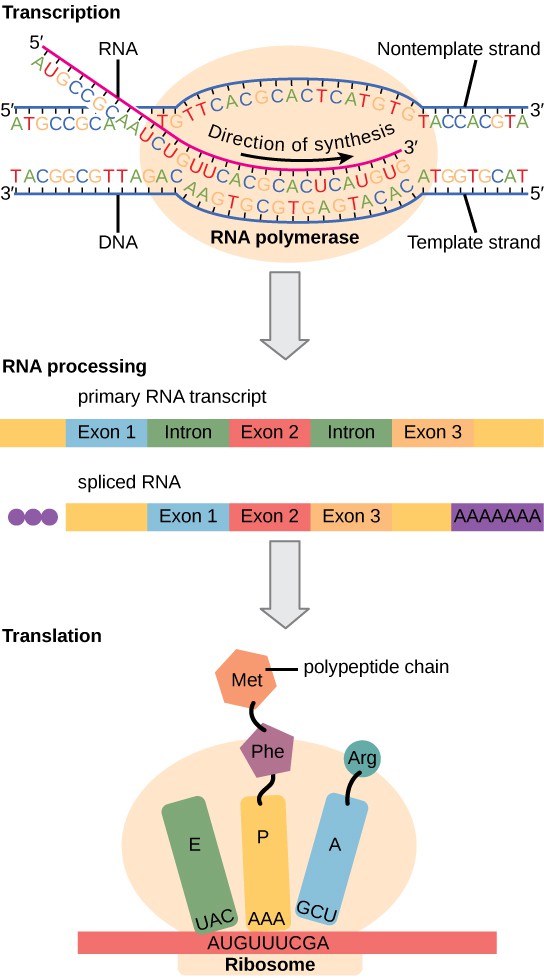
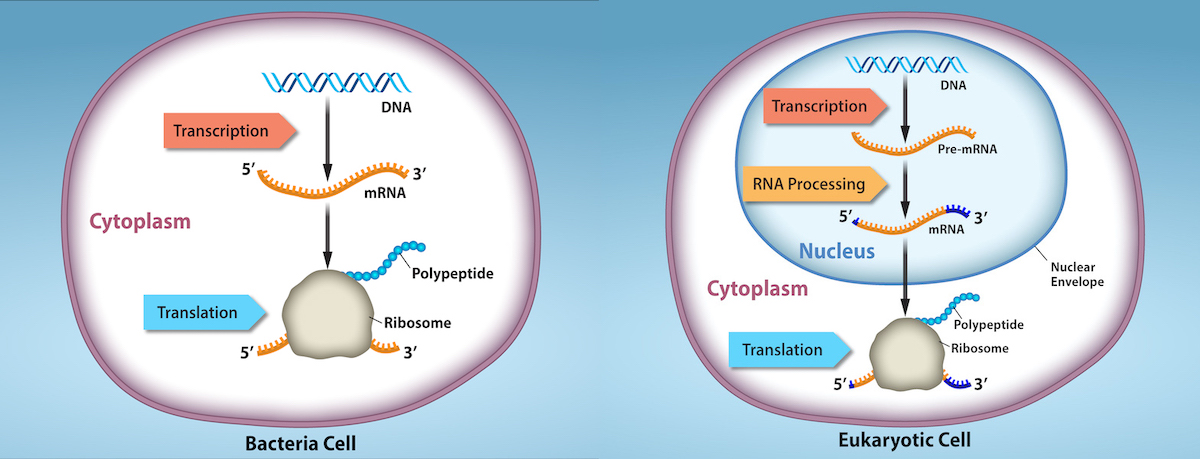
To understand how gene expression is regulated, it is important to first distinguish how a gene is expressed in prokaryotic cells versus eukaryotic cells. The overall processes of gene expression (transcription of DNA to RNA and translation of RNA to protein) are similar in prokaryotes and eukaryotes, but the cellular organization leads to key differences in when and where these processes occur.
In prokaryotes, which lack a nucleus, transcription and translation are tightly coupled in both time and location. As soon as an mRNA begins to be synthesized from DNA, ribosomes can attach and start translating it into protein. In other words, RNA transcription and protein translation occur almost simultaneously in the cytoplasm. When a needed protein has been produced in sufficient quantity, the cell simply stops transcribing the corresponding gene, and because transcription and translation are linked, protein production ceases immediately. As a result, the primary (and often only) level of gene regulation in prokaryotic cells is at the transcriptional level. By increasing or decreasing the transcription of a gene, a prokaryote directly controls how much of that gene’s protein product is present.
A classic example of prokaryotic gene regulation is the lac operon in the bacteria Escherichia coli. An operon is a cluster of genes under the control of a single promoter and regulatory region, allowing coordinated expression of genes with related functions. The lac operon contains three genes that encode enzymes for lactose metabolism, plus an adjacent promoter (binding site for RNA polymerase) and an operator (a DNA segment that a repressor protein can bind to). When lactose is absent in the environment, a repressor protein binds to the operator sequence and blocks RNA polymerase from transcribing the lactose-metabolism genes. In this state, transcription of the lac operon is effectively turned off (only tiny amounts of the enzymes are made). When lactose is present, however, lactose (or a derivative of it) binds to the repressor protein, causing the repressor to release from the operator. Freed from repression, RNA polymerase can bind the promoter and transcribe the three lactose-utilization genes at a high level, enabling the bacterium to produce the enzymes needed to import and break down lactose. This on-off control of the lac operon exemplifies how prokaryotes regulate gene expression in response to their nutritional environment, by controlling transcription initiation through regulatory proteins (repressors or activators) that respond to small molecules.
In eukaryotes, the presence of a nucleus and other organelles introduces additional levels of complexity to gene expression. Transcription occurs in the nucleus, where DNA is housed, and the produced pre-mRNA undergoes processing (such as splicing, capping, and addition of a poly-A tail) before it exits to the cytoplasm. Only after export to the cytoplasm is the mRNA translated by ribosomes into protein. Because transcription and translation are physically separated by the nuclear membrane, eukaryotes can regulate gene expression at multiple stages: before transcription (through chromatin structure), after transcription (RNA processing and stability), during translation, and even after a protein is made (through protein modifications or degradation).
The differences in the regulation of gene expression between prokaryotes and eukaryotes are summarized in Table 1 and Figure 2.
| Characteristic | Prokaryotes | Eukaryotes |
|---|---|---|
| Cell nucleus | No nucleus; DNA in cytoplasm | Nucleus present; DNA contained within nucleus |
| Timing of
transcription & translation |
Transcription and translation are coupled (occur almost simultaneously in the cytoplasm) | Transcription occurs in nucleus; mRNA must be processed and exported. Translation occurs later, in cytoplasm (separate from transcription) |
| mRNA processing | Little or none required (no introns; mRNA is used directly for translation) | Processing of pre-mRNA (addition of 5′ cap, poly-A tail, splicing to remove introns and join exons) is required before mRNA is mature |
| Primary level of gene regulation | Transcriptional control (genes mostly regulated by turning transcription on/off) | Multiple levels: epigenetic (chromatin structure), transcriptional, post-transcriptional, translational, and post-translational control |
Evolutionary Connection
Prokaryotic cells can only regulate gene expression by controlling the amount of transcription. As eukaryotic cells evolved, the complexity of the control of gene expression increased. For example, with the evolution of eukaryotic cells came compartmentalization of important cellular components and cellular processes. A nuclear region that contains the DNA was formed. Transcription and translation were physically separated into two different cellular compartments. It therefore became possible to control gene expression by regulating transcription in the nucleus, and also by controlling the RNA levels and protein translation present outside the nucleus.
Most gene regulation is done to conserve cell resources. However, other regulatory processes may be defensive. Cellular processes such as gene silencing developed to protect the cell from viral or parasitic infections. If the cell could quickly shut off gene expression for a short period of time, it would be able to survive an infection when other organisms could not. Therefore, the organism evolved a new process that helped it survive, and it was able to pass this new development to offspring.
Eukaryotic Epigenetic Regulation
Epigenetic changes are inheritable changes in gene expression that do not result from changes in the DNA sequence. Eukaryotic gene expression begins with control of access to the DNA. Transcriptional access to the DNA can be controlled in two general ways: chromatin remodeling and DNA methylation. Chromatin remodeling changes the way that DNA is associated with chromosomal histones. DNA methylation is associated with developmental changes and gene silencing.
The human genome encodes over 20,000 genes, with hundreds to thousands of genes on each of the 23 human chromosomes. The DNA in the nucleus is precisely wound, folded, and compacted into chromosomes so that it will fit into the nucleus. It is also organized so that specific segments can be accessed as needed by a specific cell type.
The first level of organization, or packing, is the winding of DNA strands around histone proteins. The DNA is wrapped around eight histone proteins to form structural units called nucleosome complexes, which can control the access of proteins to the DNA regions (Figure 3a). Under the electron microscope, this winding of DNA around histone proteins to form nucleosomes looks like small beads on a string (Figure 3b).
These beads (histone proteins) can move along the string (DNA) to expose different sections of the molecule. If DNA encoding a specific gene is to be transcribed into RNA, the nucleosomes surrounding that region of DNA can slide down the DNA to open that specific chromosomal region and allow for the transcriptional machinery (RNA polymerase) to initiate transcription (Figure 4).
How closely the histone proteins associate with the DNA is regulated by signals found on both the histone proteins and on the DNA. These signals are functional groups added to histone proteins or to DNA and determine whether a chromosomal region should be open or closed (Figure 4 depicts modifications to histone proteins and DNA). These tags are not permanent, but may be added or removed as needed. Some chemical groups (phosphate, methyl, or acetyl groups) are attached to specific amino acids in histone “tails” at the N-terminus of the protein. These groups do not alter the DNA base sequence, but they do alter how tightly wound the DNA is around the histone proteins. DNA is a negatively charged molecule and unmodified histones are positively charged; therefore, changes in the charge of the histone will change how tightly wound the DNA molecule will be. By adding chemical modifications like acetyl groups, the charge becomes less positive, and the binding of DNA to the histones is relaxed. Altering the location of nucleosomes and the tightness of histone binding opens some regions of chromatin to transcription and closes others.
The DNA molecule itself can also be modified by methylation. DNA methylation occurs within very specific regions called CpG islands. These are stretches with a high frequency of cytosine and guanine dinucleotide DNA pairs (CG) found in the promoter regions of genes. The cytosine member of the CG pair can be methylated (a methyl group is added). Methylated genes are usually silenced, although methylation may have other regulatory effects. In some cases, genes that are silenced during the development of the gametes of one parent are transmitted in their silenced condition to the offspring. Such genes are said to be imprinted. Parental diet or other environmental conditions may also affect the methylation patterns of genes, which in turn modifies gene expression. Changes in chromatin organization interact with DNA methylation. DNA methyltransferases appear to be attracted to chromatin regions with specific histone modifications. Highly methylated (hypermethylated) DNA regions with deacetylated histones are tightly coiled and transcriptionally inactive.
Epigenetic changes are not permanent, although they often persist through multiple rounds of cell division and may even cross generational lines. Chromatin remodeling alters the chromosomal structure (open or closed) as needed. If a gene is to be transcribed, the histone proteins and DNA in the chromosomal region encoding that gene are modified in a way that opens the promoter region to allow RNA polymerase and other proteins, called transcription factors, to bind and initiate transcription. If a gene is to remain turned off, or silenced, the histone proteins and DNA have different modifications that signal a closed chromosomal configuration. In this closed configuration, the RNA polymerase and transcription factors do not have access to the DNA and transcription cannot occur (Figure 5).
Post-transcriptional Eukaryotic RNA Regulation: mRNA Splicing
The newly transcribed eukaryotic mRNAs must undergo several processing steps before they can be transferred from the nucleus to the cytoplasm and translated into a protein. The additional steps involved in eukaryotic mRNA maturation create a molecule that is much more stable than a prokaryotic mRNA. For example, eukaryotic mRNAs last for several hours, whereas the typical prokaryotic mRNA lasts no more than five seconds.
The mRNA transcript is first coated in RNA-stabilizing proteins to prevent it from degrading while it is processed and exported out of the nucleus. This occurs while the pre-mRNA still is being synthesized by adding a special nucleotide “cap” to the 5′ end of the growing transcript. In addition to preventing degradation, factors involved in protein synthesis recognize the cap to help initiate translation by ribosomes.
Once elongation is complete, an enzyme then adds a string of approximately 200 adenine residues to the 3′ end, called the poly-A tail. This modification further protects the pre-mRNA from degradation and signals to cellular factors that the transcript needs to be exported to the cytoplasm.
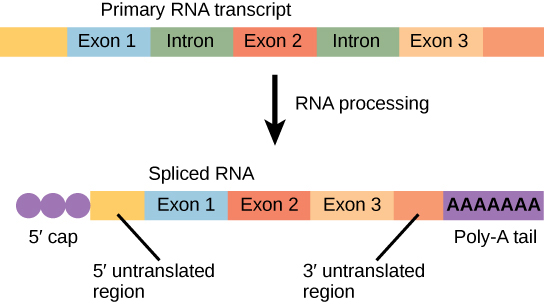
Eukaryotic genes are composed of protein-coding sequences called exons (ex-on signifies that they are expressed) and intervening sequences called introns (int-ron denotes their intervening role). Introns are removed from the pre-mRNA during processing. Intron sequences in mRNA do not encode functional proteins. It is essential that all of a pre-mRNA’s introns be completely and precisely removed before protein synthesis so that the exons join together to code for the correct amino acids. If the process errs by even a single nucleotide, the sequence of the rejoined exons would be shifted, and the resulting protein would be nonfunctional. The process of removing introns and reconnecting exons is called splicing (Figure 6). Introns are removed and degraded while the pre-mRNA is still in the nucleus.
Alternative RNA Splicing
In the 1970s, genes were first observed that exhibited alternative RNA splicing. Alternative RNA splicing is a mechanism that allows different protein products to be produced from one gene when different combinations of introns (and sometimes exons) are removed from the transcript (Figure 7). This alternative splicing can be haphazard, but more often it is controlled and acts as a mechanism of gene regulation, with the frequency of different splicing alternatives controlled by the cell as a way to control the production of different protein products in different cells, or at different stages of development. Alternative splicing is now understood to be a common mechanism of gene regulation in eukaryotes; according to one estimate, 70% of genes in humans are expressed as multiple proteins through alternative splicing.
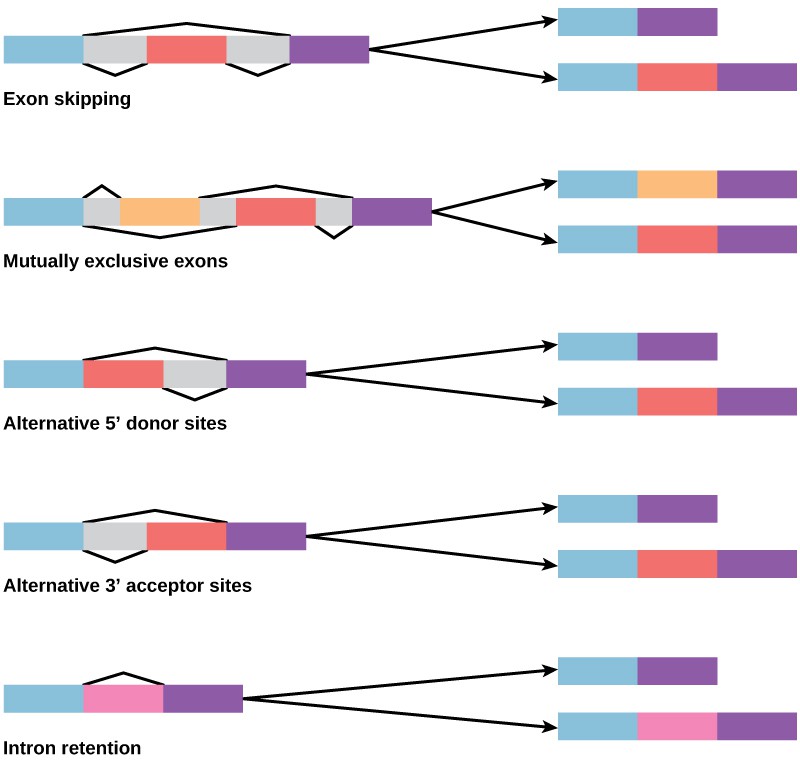
How could alternative splicing evolve? Introns have a beginning and ending recognition sequence, and it is easy to imagine the failure of the splicing mechanism to identify the end of an intron and find the end of the next intron, thus removing two introns and the intervening exon. In fact, there are mechanisms in place to prevent such exon skipping, but mutations are likely to lead to their failure. Such “mistakes” would more than likely produce a nonfunctional protein. Indeed, the cause of many genetic diseases is alternative splicing rather than mutations in a sequence. However, alternative splicing would create a protein variant without the loss of the original protein, opening up possibilities for adaptation of the new variant to new functions. Gene duplication has played an important role in the evolution of new functions in a similar way—by providing genes that may evolve without eliminating the original functional protein.
Gene Regulatory Networks
In the previous section, we discussed how alternative splicing allows a single gene to produce multiple proteins by rearranging its exons in different combinations. This is just one mechanism by which gene expression can be fine-tuned. However, gene regulation is rarely a simple, linear process. In most cases, the expression of a gene is influenced by many factors beyond splicing, such as signals from other genes, proteins, and even environmental cues. This interconnectedness is part of a larger system known as a gene regulatory network (GRN), where genes and their products interact to control the timing, location, and intensity of gene expression.
A gene regulatory network functions much like a complex communication system within cells, ensuring that genes are expressed only when needed. As part of this system, the expression of one gene can influence another. Gene products (e.g., mRNA, proteins) that promote the expression of other genes are broadly referred to as activators. For example, the protein product of one gene might bind to regulatory region of another gene, enhancing the production of mRNA. In contrast, gene products that inhibit the expression of other genes are broadly referred to as repressors. These interactions form feedback loops and pathways, coordinating gene activity across the cell or even throughout the organism. The result is a carefully regulated balance of gene expression, allowing cells to adapt to changes and maintain proper function.
What makes gene regulatory networks particularly powerful is their ability to integrate signals from both within the genome and the surrounding environment. For instance, in response to stress or a lack of nutrients, a cell might activate a set of genes that help it survive, while repressing others that aren’t immediately needed. Similarly, during development, a cascade of gene interactions ensures that cells differentiate into specialized types, such as muscle or nerve cells, at the right time and place.
When two genes interaction to affect the expression one another, the phenomenon is referred to as epistasis. Epistasis is common feature of gene regulatory networks. The interaction of two genes, however, can depend on the specific alleles (gene variants) present in an individual’s genome. One allele may modify or completely mask the expression of another gene, while a different allele for the same gene may have no affect at all. Such allelelic-specific interactions help explain why certain traits, like disease susceptibility, may not follow straightforward patterns of inheritance, as the combined effects of different alleles play a key role in determining the final outcome.
Gene expression is not just the product of an individual gene but the result of a vast and interconnected network of genes, proteins, and environmental factors working together. Gene regulatory networks represent the complexity and precision of biological systems, coordinating the expression of genes in response to internal and external signals to ensure that organisms function properly. Understanding these networks is key to unlocking the full picture of how genes influence traits, behaviors, and even responses to environmental changes.
See the following article for a review of gene expression and regulation.
Glossary
- activators
- instigate positive expression of a gene
alternative RNA splicing
a post-transcriptional gene regulation mechanism in eukaryotes in which multiple protein products are produced by a single gene through alternative splicing combinations of the RNA transcript
- epigenetic
- describing non-genetic regulatory factors, such as changes in modifications to histone proteins and DNA that control accessibility to genes in chromosomes
- epistasis
- when the expression of one gene is modified (e.g., masked, inhibited or suppressed) by the expression of another gene.
gene expression
processes that control whether a gene is expressed
- gene regulatory network
- a collection of molecular regulators that control gene expression levels in a cell
- post-transcriptional
- control of gene expression after the RNA molecule has been created but before it is translated into protein
- post-translational
- control of gene expression after a protein has been created
-
- repressors
- instigate negative expression of a gene
References and Resources
- Biology LibreTexts. (2025). 16.2: Eukaryotic Epigenetic Regulation. Retrieved from https://bio.libretexts.org
- LibreTexts. (2025). 23.2: Eukaryotic Epigenetic Gene Regulation. In Biology LibreTexts. Retrieved August 29, 2025
- Fowler, S., Roush, R., & Wise, J. (2013). Concepts of Biology. OpenStax.
- OpenStax. (2018). Biology 2e. OpenStax.
- Pressbooks Hawaii (Leeward CC). (2023). How Genes Are Regulated (adapted OpenStax content).
- Reed, S. M., & Quelle, D. E. (2015). p53 Acetylation: Regulation and consequences. Cancers, 7(1), 30–69. https://doi.org/10.3390/cancers7010030