10 Biotechnology
Learning Objectives
- Describe gel electrophoresis
- Explain molecular and reproductive cloning
- Describe biotechnology uses in medicine and agriculture
- Explain how the polymerase chain reaction works to make copies of DNA segments
- Explain how recombinant DNA is assembled and used to modify genomes
- Explain how the CRISPR-Cas9 system differs from other forms of gene editing
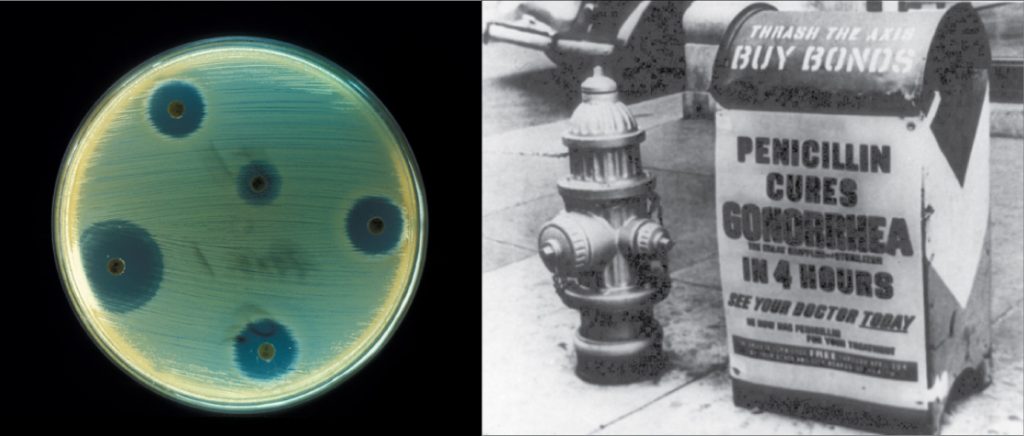
Biotechnology is the use of biological agents for technological advancement. Biotechnology was used for breeding livestock and crops long before people understood the scientific basis of these techniques. Since the discovery of the structure of DNA in 1953, the biotechnology field has grown rapidly through both academic research and private companies. The primary applications of this technology are in medicine (vaccine and antibiotic production) and agriculture (crop genetic modification in order to increase yields). Biotechnology also has many industrial applications, such as fermentation, treating oil spills, and producing biofuels (Figure 1).
Basic Techniques to Manipulate Genetic Material (DNA and RNA)
To understand the basic techniques used to work with nucleic acids, remember that nucleic acids are macromolecules made of nucleotides (a sugar, a phosphate, and a nitrogenous base) linked by phosphodiester bonds. The phosphate groups on these molecules each have a net negative charge. An entire set of DNA molecules in the nucleus is called the genome. DNA has two complementary strands linked by hydrogen bonds between the paired bases. Exposure to high temperatures (DNA denaturation) can separate the two strands and cooling can reanneal them. The DNA polymerase enzyme can replicate the DNA. Unlike DNA, which is located in the eukaryotic cells’ nucleus, RNA molecules leave the nucleus. The most common type of RNA that researchers analyze is the messenger RNA (mRNA) because it represents the protein-coding genes that are actively expressed. However, RNA molecules present some other challenges to analysis, as they are often less stable than DNA.
DNA and RNA Extraction
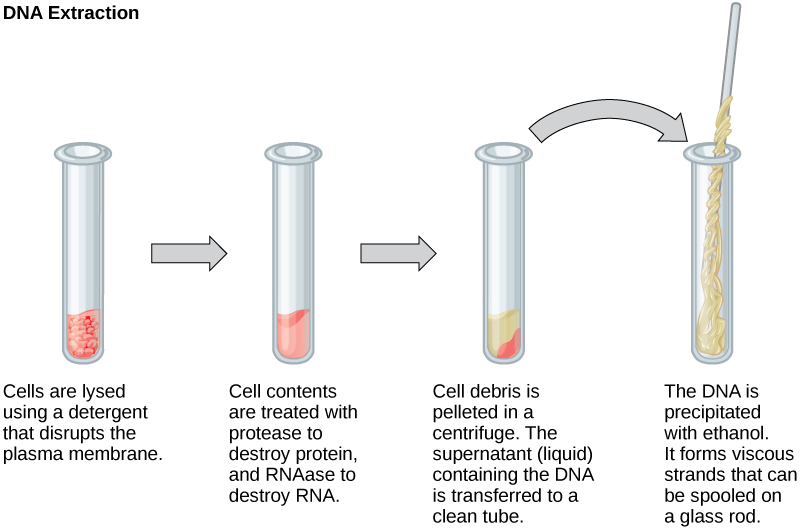
To study or manipulate nucleic acids, one must first isolate or extract the DNA or RNA from the cells. Researchers use various techniques to extract different types of DNA (Figure 2). Most nucleic acid extraction techniques involve steps to break open the cell and use enzymatic reactions to destroy all macromolecules that are not desired (such as unwanted molecule degradation and separation from the DNA sample). A lysis buffer (a solution which is mostly a detergent) breaks cells. Note that lysis means “to split”. These enzymes break apart lipid molecules in the cell membranes and nuclear membranes. Enzymes such as proteases that break down proteins inactivate macromolecules, and ribonucleases (RNAses) that break down RNA. Using alcohol precipitates the DNA. Human genomic DNA is usually visible as a gelatinous, white mass. One can store the DNA samples frozen at –80°C for several years.
Scientists perform RNA analysis to study gene expression patterns in cells. RNA is naturally very unstable because RNAses are commonly present in nature and very difficult to inactivate. Similar to DNA, RNA extraction involves using various buffers and enzymes to inactivate macromolecules and preserve the RNA.
Gel Electrophoresis
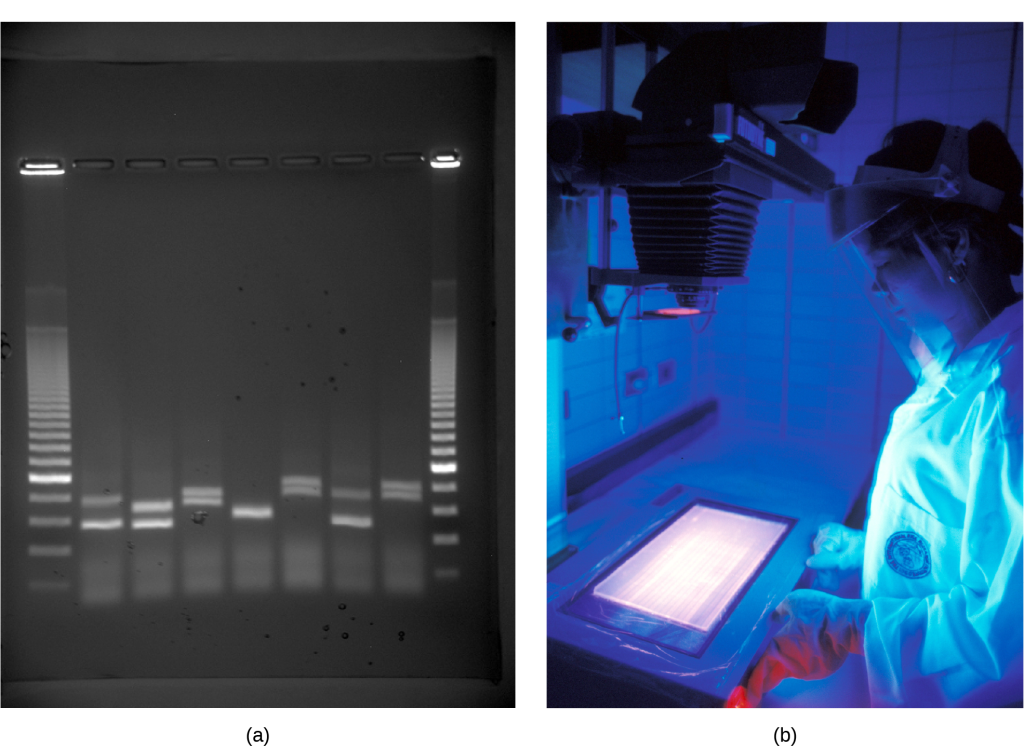
Because nucleic acids are negatively charged ions at neutral or basic pH in an aqueous environment, an electric field can mobilize them. Gel electrophoresis is a technique that scientists use to separate molecules on the basis of size, using this charge. One can separate the nucleic acids as whole chromosomes or fragments. The nucleic acids load into a slot near the semisolid, porous gel matrix’s negative electrode, and pulled toward the positive electrode at the gel’s opposite end. Smaller molecules move through the gel’s pores faster than larger molecules. This difference in the migration rate separates the fragments on the basis of size. There are molecular weight standard samples that researchers can run alongside the molecules to provide a size comparison. We can observe nucleic acids in a gel matrix using various fluorescent or colored dyes. Distinct nucleic acid fragments appear as bands at specific distances from the gel’s top (the negative electrode end) on the basis of their size (Figure 3). A mixture of genomic DNA fragments of varying sizes appear as a long smear; whereas, uncut genomic DNA is usually too large to run through the gel and forms a single large band at the gel’s top.
Nucleic Acid Fragment Amplification by Polymerase Chain Reaction
Although genomic DNA is visible to the naked eye when it is extracted in bulk, DNA analysis often requires focusing on one or more specific genome regions. Polymerase chain reaction (PCR) is a technique that scientists use to amplify specific DNA regions for further analysis (Figure 4). Researchers use PCR for many purposes in laboratories, such as cloning gene fragments to analyze genetic diseases, identifying contaminant foreign DNA in a sample, and amplifying DNA for sequencing. More practical applications include determining paternity and detecting genetic diseases.
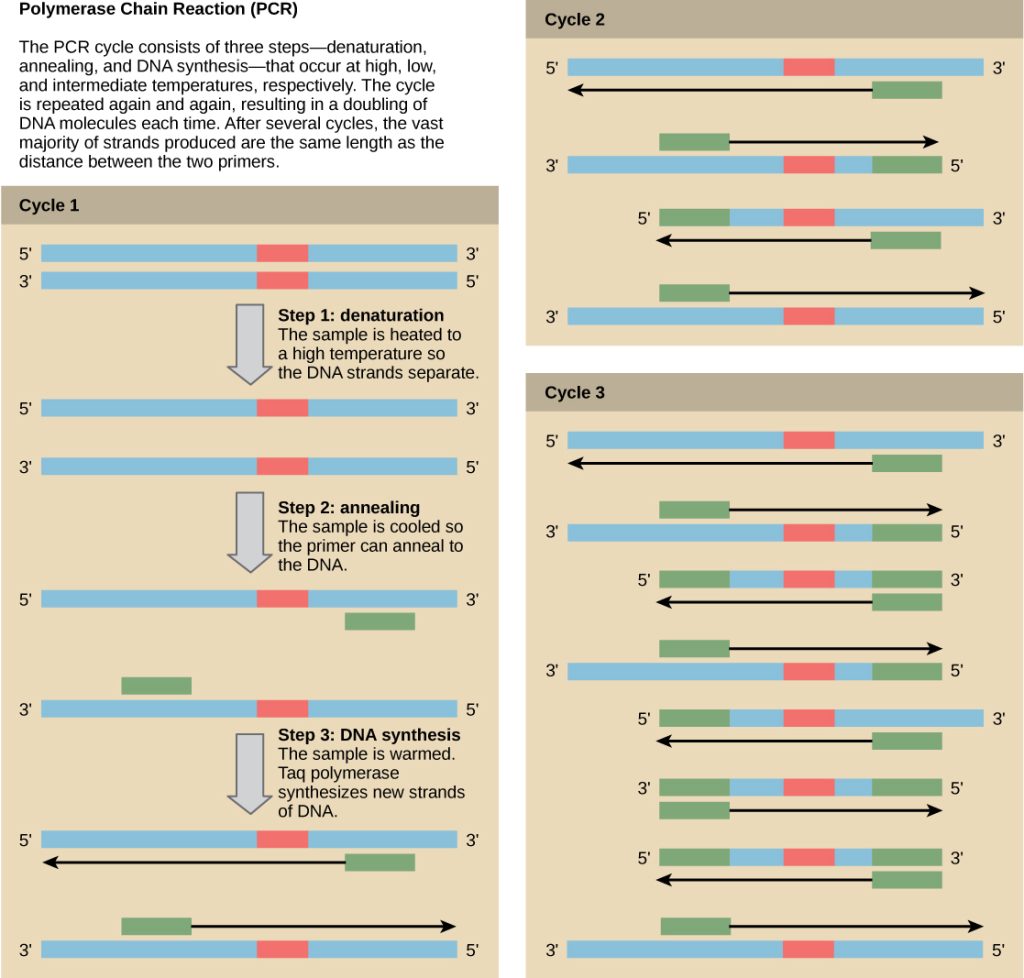
DNA fragments can also be amplified from an RNA template in a process called reverse transcriptase PCR (RT-PCR). The first step is to recreate the original DNA template strand (called cDNA) by applying DNA nucleotides to the mRNA. This process is called reverse transcription. This requires the presence of an enzyme called reverse transcriptase. After the cDNA is made, regular PCR can be used to amplify it.
Molecular Cloning
Lecture Video: Genetic engineering, recombinant DNA, and gene therapy.
In general, the word “cloning” means the creation of a perfect replica; however, in biology, the re-creation of a whole organism is referred to as “reproductive cloning.” Long before attempts were made to clone an entire organism, researchers learned how to reproduce desired regions or fragments of the genome, a process that is referred to as molecular cloning.
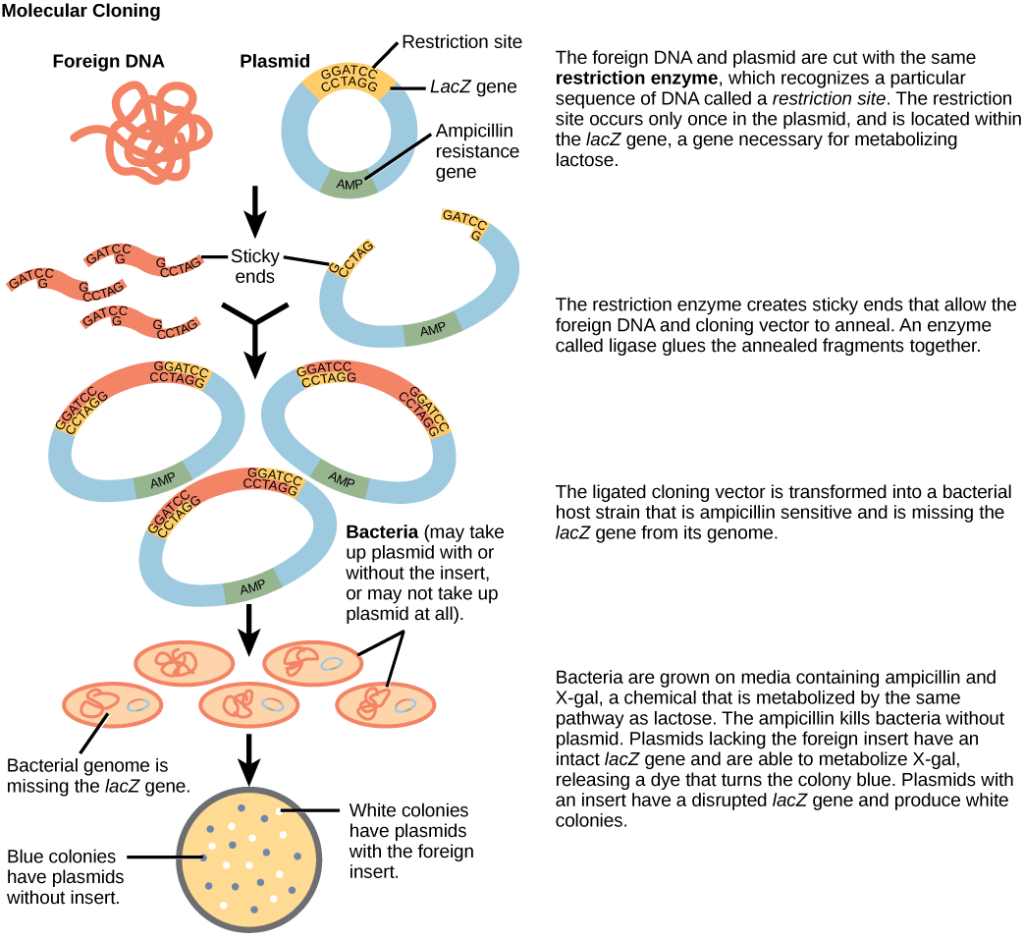
Cloning small genome fragments allows researchers to manipulate and study specific genes (and their protein products), or noncoding regions in isolation. A plasmid, or vector, is a small circular DNA molecule that replicates independently of the chromosomal DNA. In cloning, scientists can use the plasmid molecules to provide a “folder” in which to insert a desired DNA fragment. Plasmids are usually introduced into a bacterial host for proliferation. In the bacterial context, scientists call the DNA fragment from the human genome (or the genome of another studied organism) foreign DNA, or a transgene, to differentiate it from the bacterium’s DNA, or the host DNA.
Plasmids occur naturally in bacterial populations (such as Escherichia coli) and have genes that can contribute favorable traits to the organism, such as antibiotic resistance (the ability to be unaffected by antibiotics). Scientists have repurposed and engineered plasmids as vectors for molecular cloning and the large-scale production of important reagents, such as insulin and human growth hormone. An important feature of plasmid vectors is the ease with which scientists can introduce a foreign DNA fragment via the multiple cloning site (MCS). The MCS is a short DNA sequence containing multiple sites that different commonly available restriction endonucleases can cut. Restriction endonucleases recognize specific DNA sequences and cut them in a predictable manner. They are naturally produced by bacteria as a defense mechanism against foreign DNA. Many restriction endonucleases make staggered cuts in the two DNA strands, such that the cut ends have a 2- or 4-base single-stranded overhang. Because these overhangs are capable of annealing with complementary overhangs, we call them “sticky ends.” Adding the enzyme DNA ligase permanently joins the DNA fragments via phosphodiester bonds. In this way, scientists can splice any DNA fragment generated by restriction endonuclease cleavage between the plasmid DNA’s two ends that has been cut with the same restriction endonuclease (Figure 5).
Recombinant DNA Molecules
Plasmids with foreign DNA inserted into them are called recombinant DNA molecules because they are created artificially and do not occur in nature. They are also called chimeric molecules because the origin of different molecule parts of the molecules can be traced back to different species of biological organisms or even to chemical synthesis. We call proteins that are expressed from recombinant DNA molecules recombinant proteins. Not all recombinant plasmids are capable of expressing genes. The recombinant DNA may need to move into a different vector (or host) that is better designed for gene expression. Scientists may also engineer plasmids to express proteins only when certain environmental factors stimulate them, so they can control the recombinant proteins’ expression.
VISUAL CONNECTION
You are working in a molecular biology lab and, unbeknownst to you, your lab partner left the foreign genomic DNA that you are planning to clone on the lab bench overnight instead of storing it in the freezer. As a result, it was degraded by nucleases, but still used in the experiment. The plasmid, on the other hand, is fine. What results would you expect from your molecular cloning experiment?
- There will be no colonies on the bacterial plate.
- There will be blue colonies only.
- There will be blue and white colonies.
- There will be white colonies only.
Answer:
You’d expect the experiment would result in blue colonies only. (b.)
CRISPR Technology
CRISPR technology has transformed the field of genetic engineering by providing a precise, efficient, and versatile method for editing genomes. At the heart of this innovation is the CRISPR-Cas9 system, a powerful tool derived from a natural defense mechanism found in bacteria (Figure 6).
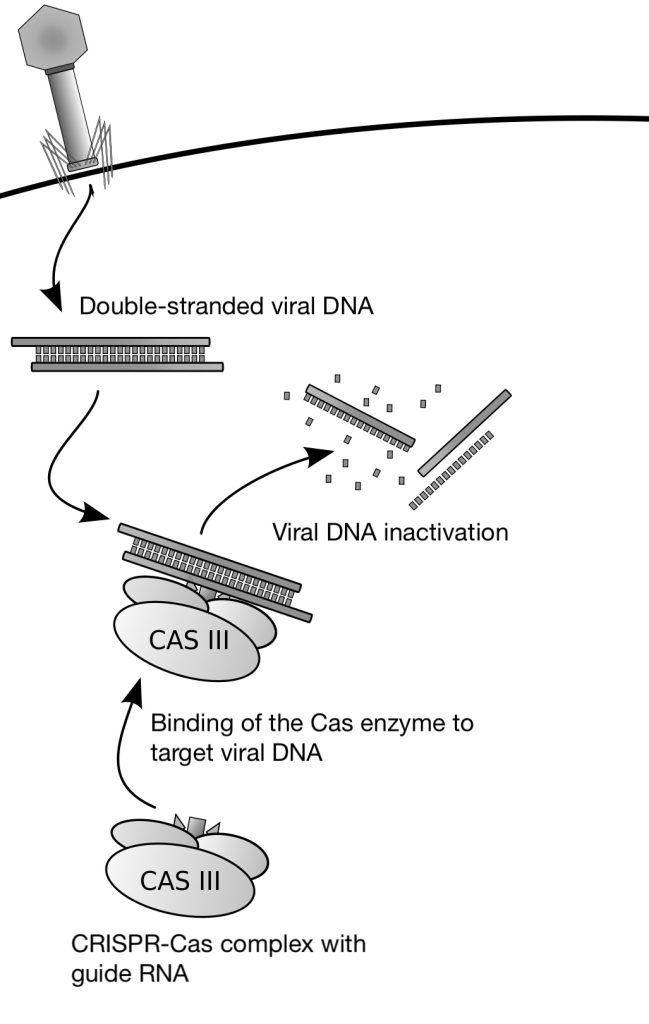
CRISPR-Cas9 stands for Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) associated with the Cas9 protein. This system uses a nuclease enzyme (Cas9) guided by RNA molecules to introduce breaks at specific DNA sequences. For Cas9 to bind and cut effectively, the target sequence must be located next to a short DNA motif called the Protospacer Adjacent Motif (PAM), which is required for recognition.
The essential elements of this system are a single guide RNA (sgRNA) that homes in on the target sequence and a nuclease that can make a cut in the sequence bound by the guide RNA. By engineering guide RNAs complementary to a target gene, it is possible to target the nuclease to edit the gene in situ. In the CRISPR-Cas9 system, the Cas9 endonuclease cuts both strands of the gene sequence targeted by the guide RNA. This generates a double-strand break that the cell attempts to repair (Figure 7).
Double-strand breaks in DNA can be repaired by simple, nonhomologous end joining (NHEJ) or by Homology Directed Repair (HDR). When a break is fixed by NHEJ, there is a good chance that deletions or insertions will occur, often inactivating the gene. Thus, targeted cleavage of a site by CRISPR-Cas9 can easily and specifically inactivate a gene, making it simple to characterize the gene’s function.
But, what if you wished to mutate the gene at a specific site to study the effect of the mutation? This can be achieved through homology directed repair (HDR). In HDR, a repair template DNA is provided bearing the desired change (mutation) to the target sequence. After Cas9 cuts the target sequence, the template DNA is used to repair the cut, thereby inserting the new mutation. In the same way, it is possible to correct a deleterious mutation in the genome by cleaving at the appropriate spot and providing the correct sequence as repair template DNA for HDR. The simplicity of the system is what makes CRISPR-Cas9 such a powerful gene editing tool.
LINK TO LEARNING
- Learn more about CRISPR-Cas9 using the following online interactive tool: HHMI BioInteractive CRISPR-Cas9 Interactive module
- Read more about how CRISPR-Cas9 works in the following article: “A simple guide to CRISPR, one of the biggest science stories of the decade” (Vox)
- Listen to the following interview with Dr. Jennifer Donda, the Nobel Prize winning co-discovers of CRISPR: Jennifer Doudna and the Power of CRISPR (Clear + Vivid)
VISUAL CONNECTION
Video: Genetic Engineering Will Change Everything Forever – CRISPR
Biotechnology in Medicine and Agriculture
It is easy to see how biotechnology can be used for medicinal purposes. Knowledge of the genetic makeup of our species, the genetic basis of heritable diseases, and the invention of technology to manipulate and fix mutant genes provides methods to treat the disease. Biotechnology in agriculture can enhance resistance to disease, pest, and environmental stress, and improve both crop yield and quality.
Genetic Diagnosis and Gene Therapy
Scientists call the process of testing for suspected genetic defects before administering treatment genetic diagnosis by genetic testing. Depending on the inheritance patterns of a disease-causing gene, family members are advised to undergo genetic testing. For example, doctors usually advise women diagnosed with breast cancer to have a biopsy so that the medical team can determine the genetic basis of cancer development. Doctors base treatment plans on genetic test findings that determine the type of cancer. If inherited gene mutations cause the cancer, doctors also advise other female relatives to undergo genetic testing and periodic screening for breast cancer. Doctors also offer genetic testing for fetuses (or embryos with in vitro fertilization) to determine the presence or absence of disease-causing genes in families with specific debilitating diseases.
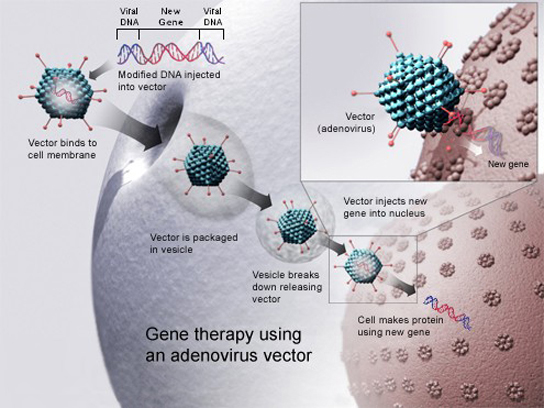
Gene therapy is a genetic engineering technique used to cure disease. In its simplest form, it involves the introduction of a good gene at a random location in the genome to aid the cure of a disease that is caused by a mutated gene. The good gene is usually introduced into diseased cells as part of a vector transmitted by a virus that can infect the host cell and deliver the foreign DNA (Figure 8). More advanced forms of gene therapy try to correct the mutation at the original site in the genome, such as is the case with treatment of severe combined immunodeficiency (SCID).
Production of Vaccines, Antibiotics, and Hormones
Traditional vaccination strategies use weakened or inactive forms of microorganisms to mount the initial immune response. Modern techniques use the genes of microorganisms cloned into vectors to mass produce the desired antigen. Doctors then introduce the antigen into the body to stimulate the primary immune response and trigger immune memory. The medical field has used genes cloned from the influenza virus to combat the constantly changing strains of this virus.
Antibiotics are a biotechnological product. Microorganisms, such as fungi, naturally produce them to attain an advantage over bacterial populations. Cultivating and manipulating fungal cells produces antibodies.
Scientists used recombinant DNA technology to produce large-scale quantities of human insulin in E. coli as early as 1978. Previously, it was only possible to treat diabetes with pig insulin, which caused allergic reactions in humans because of differences in the gene product. In addition, doctors use human growth hormone (HGH) to treat growth disorders in children. Researchers cloned the HGH gene from a cDNA library and inserted it into E. coli cells by cloning it into a bacterial vector.
GMOs and Transgenic Organisms
Genetically Modified Organisms (GMOs) and transgenic organisms are vital in biotechnology for the production of complex proteins and for research purposes. While bacteria are commonly used to produce recombinant proteins, certain proteins or medical applications require modification of eukaryotic genomes. The term GMO refers to any organism, prokaryotic or eukaryotic, whose genome has been genetically altered. This could many having an existing gene deactivated or modified. Transgenic organisms are a type of GMO that have specifically had genetic material from another species edited into their genome, allowing them to express new traits or produce substances not naturally found in their species.
VISUAL CONNECTION
Video: Are GMOs Good or Bad? Genetic Engineering & Our Food
Manipulating the DNA of plants (i.e., creating GMOs) has helped to create desirable traits, such as disease resistance, herbicide and pesticide resistance, better nutritional value, and better shelf-life (Figure 9). Plants are the most important source of food for the human population. Farmers developed ways to select for plant varieties with desirable traits long before modern-day biotechnology practices were established.

We call plants that have received recombinant DNA from other species transgenic plants. Because they are not natural, government agencies closely monitor transgenic plants and other GMOs to ensure that they are fit for human consumption and do not endanger other plant and animal life. Because foreign genes can spread to other species in the environment, extensive testing is required to ensure ecological stability. Staples like corn, potatoes, and tomatoes were the first crop plants that scientists genetically engineered.
Transformation of Plants Using Agrobacterium tumefaciens
Gene transfer occurs naturally between species in microbial populations. Many viruses that cause human diseases, such as cancer, act by incorporating their DNA into the human genome. In plants, tumors caused by the bacterium Agrobacterium tumefaciens occur by DNA transfer from the bacterium to the plant. Although the tumors do not kill the plants, they stunt the plants and they become more susceptible to harsh environmental conditions. A. tumefaciens affects many plants such as walnuts, grapes, nut trees, and beets. Artificially introducing DNA into plant cells is more challenging than in animal cells because of the thick plant cell wall.
Researchers used the natural transfer of DNA from Agrobacterium to a plant host to introduce DNA fragments of their choice into plant hosts. In nature, the disease-causing A. tumefaciens have a set of plasmids, Ti plasmids (tumor-inducing plasmids), that contain genes to produce tumors in plants. DNA from the Ti plasmid integrates into the infected plant cell’s genome. Researchers manipulate the Ti plasmids to remove the tumor-causing genes and insert the desired DNA fragment for transfer into the plant genome. The Ti plasmids carry antibiotic resistance genes to aid selection and researchers can propagate them in E. coli cells as well.
The Organic Insecticide Bacillus thuringiensis
Bacillus thuringiensis (Bt) is a bacterium that produces protein crystals during sporulation that are toxic to many insect species that affect plants. Insects need to ingest Bt toxin in order to activate the toxin. Insects that have eaten Bt toxin stop feeding on the plants within a few hours. After the toxin activates in the insects’ intestines, they die within a couple of days. Modern biotechnology has allowed plants to encode their own crystal Bt toxin that acts against insects. Scientists have cloned the crystal toxin genes from Bt and introduced them into plants. Bt toxin is safe for the environment, nontoxic to humans and other mammals, and organic farmers have approved it as a natural insecticide.
Flavr Savr Tomato
The first GM crop on the market was the Flavr Savr Tomato in 1994. Scientists used antisense RNA technology to slow the softening and rotting process caused by fungal infections, which led to increased shelf life of the GM tomatoes. Additional genetic modification improved the tomato’s flavor. The Flavr Savr tomato did not successfully stay in the market because of problems maintaining and shipping the crop.
Glossary
-
antibiotic resistance: The ability of an organism to be unaffected by an antibiotic’s actions.
biotechnology: The use of biological agents for technological advancement in medicine, agriculture, or industry.
Cas9: An endonuclease enzyme that cuts DNA at the location specified by the guide RNA in the CRISPR-Cas9 system.
CRISPR: A genetic engineering tool derived from a bacterial immune system that allows for precise, targeted modification of DNA in living organisms.
foreign DNA: DNA that originates from a different species or is artificially synthesized.
gel electrophoresis: A technique used to separate molecules based on size by applying an electric charge through a gel matrix.
gene targeting: A method of altering a specific gene’s sequence by introducing a modified version on a vector.
gene therapy: A technique used to treat genetic diseases by replacing or repairing faulty genes with functional ones.
genetic diagnosis: The process of analyzing genes to determine the potential for disease development.
genetic engineering: The alteration of an organism’s genetic makeup to achieve desired traits.
genetic testing: The process of detecting the presence or absence of disease-causing genes.
genetically modified organism (GMO): An organism whose genome has been artificially altered through biotechnology.
Homology Directed Repair (HDR): A precise DNA repair pathway that uses a homologous DNA sequence as a template to correct or introduce mutations.
host DNA: DNA naturally present in the genome of the organism of interest.
lysis buffer: A solution used to break open cell membranes and release cell contents during DNA or RNA extraction.
molecular cloning: The process of replicating DNA fragments to study or manipulate specific genes.
multiple cloning site (MCS): A short DNA sequence containing multiple restriction enzyme recognition sites used in cloning.
Nonhomologous End Joining (NHEJ): An error-prone DNA repair pathway that directly joins broken DNA ends, often causing insertions or deletions.
polymerase chain reaction (PCR): A technique that amplifies specific DNA sequences for analysis or use in biotechnology.
Protospacer Adjacent Motif (PAM): A short DNA sequence required for Cas9 recognition and binding during CRISPR editing.
probe: A small fragment of DNA or RNA used to detect complementary sequences in a sample.
protease: An enzyme that breaks down proteins by cleaving peptide bonds.
recombinant DNA: DNA molecules artificially created by combining fragments from different sources; also called chimeric DNA.
recombinant protein: A protein product expressed from recombinant DNA through cloning and expression systems.
repair template DNA: A piece of DNA provided during CRISPR editing that serves as a template for HDR to introduce or correct mutations.
reproductive cloning: The creation of an entire organism that is genetically identical to the original.
restriction endonuclease: An enzyme that recognizes specific DNA sequences and cleaves them in predictable patterns.
reverse genetics: A method for determining gene function by starting with a known gene and studying its effects.
reverse transcriptase PCR (RT-PCR): A PCR method that first converts RNA into DNA using reverse transcriptase before amplification.
ribonuclease: An enzyme that degrades RNA molecules.
single guide RNA (sgRNA): A synthetic RNA molecule that directs Cas9 to a specific DNA sequence for editing.
Target DNA: The specific DNA sequence in the genome recognized and cleaved by the CRISPR-Cas9 complex.
Ti plasmid: A plasmid derived from Agrobacterium tumefaciens that scientists use to introduce foreign DNA into plant genomes.
transgenic: An organism that has received DNA from a different species to express new traits.
Access for free at https://openstax.org/books/biology-2e/pages/1-introduction