Physics
Particles
Learning Objectives
- Be able to describe the basic structure of an an atom (protons, neutrons, electrons, orbitals)
- Be able to define and give an example of an ion
An atom is the smallest unit of matter that retains all of the chemical properties of an element. An atom consists of two regions. The first is the tiny atomic nucleus, which is in the center of the atom and contains positively charged particles called protons and neutral, uncharged, particles called neutrons. The second, much larger, region of the atom is a “cloud” of electrons, negatively charged particles that orbit around the nucleus. The attraction between the positively charged protons and negatively charged electrons holds the atom together. Most atoms contain all three of these types of subatomic particles—protons, electrons, and neutrons.
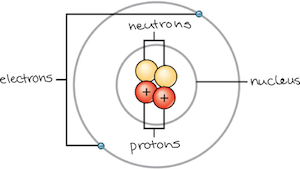
An atom is the smallest unit of matter that retains all of the chemical properties of an element. An atom consists of two regions. The first is the tiny atomic nucleus, which is in the center of the atom and contains positively charged particles called protons and neutral, uncharged, particles called neutrons. The second, much larger, region of the atom is a “cloud” of electrons, negatively charged particles that orbit around the nucleus. The attraction between the positively charged protons and negatively charged electrons holds the atom together. Most atoms contain all three of these types of subatomic particles—protons, electrons, and neutrons.
Electricity and magnetism
Learning Objectives
- Be able to define electric and magnetic fields
- Know how an electric current creates a magnetic field
So going into more depth of an electric field…the magnitude and direction of the electric field are expressed by the value of E, called electric field strength or electric field intensity or simply the electric field. Instead of considering the electric force as a direct interaction of two electric charges at a distance from each other, one charge is considered the source of an electric field that extends outward into the surrounding space, and the force exerted on a second charge in this space is considered as a direct interaction between the electric field and the second charge. The strength of an electric field E at any point may be defined as the electric, or Coulomb, force F exerted per unit positive electric charge q at that point, or simply E = F/q.
So, electric fields. Charges. Electrons. Magnets? Well how do magnets fit into all of this? Well, forces between electric currents are what cause a magnetic force. We understand that magnets have two poles and that depending on the orientation of two magnets there can be attraction (opposite poles) or repulsion (similar poles). We recognize that there is some region extending around a magnet where this happens. The magnetic field describes this region.
Particles: “Matter, elements, and atoms” by Khan Academy. Licensed CC BY-NC-SA 4.0. “Ions” by Chemistry Libretexts. Licensed CC-BY-NC-SA 4.0.
Electricity and magnetism: “Basic electrical quantities: current, voltage, power” by Willy McAllister. Licensed CC BY-NC-SA 4.0. Action Potentials – Introduction to Sensation and Perception (umn.edu) by Students of PSY 3031. Licensed CC BY 4.0

