Ozone
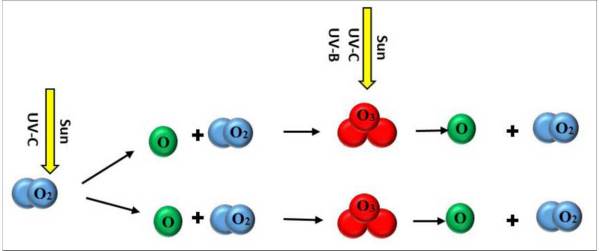
Ozone (O3) is a molecule in which three atoms of oxygen are bonded together. The oxygen gas in the air we breathe has two oxygen atoms bonded together (O2). Ozone is relatively unstable and releases its third oxygen atom readily so it oxidizes and burns things more readily than oxygen gas. This characteristic makes ozone in the troposphere (ground-level ozone) an air pollutant (see “Outdoor Air Pollution”). But in the stratosphere, ozone is essential for protecting life on Earth. Ozone in the stratosphere is formed when an oxygen molecule (O2) is broken apart into two separate oxygen atoms (O) by high-energy ultraviolet (UV) solar radiation. Each of the resulting oxygen atoms (O) in turn reacts with an oxygen molecule (O2) creating ozone (O3) (Figure 6.2). Once produced, ozone can absorb UV radiation breaking the molecule to regenerate an oxygen molecule and a single oxygen atom. So, while ozone is continually being replenished, it is also continually being destroyed. If the rate of ozone creation is equal to the rate of destruction, the total amount will remain the same. Because there is so much oxygen in our atmosphere, this “ozone-oxygen cycle” is continuously absorbing UV radiation.
The Ozone Layer
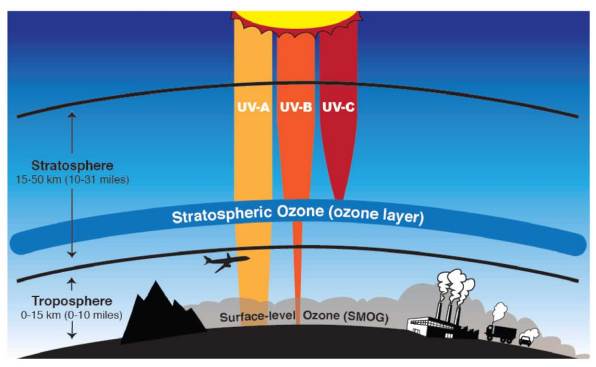
Ozone makes up a very small proportion of the gases in our atmosphere and most of it is concentrated in a portion of the stratosphere roughly 17 – 30 km above the surface. This region, called the ozone layer, acts as a protective shield that protects life on the surface of the Earth by absorbing most of the harmful portions of the high-energy UV radiation coming from the sun. UV is subdivided into three types namely UV-A, UV-B, and UV-C (Figure 6.3). Of these three types, UV-A is the least energetic and least harmful but can cause some damage to living cells, resulting in sunburns and skin damage. UV-A is also not absorbed by ozone in the stratosphere and is therefore transmitted through the atmosphere to the surface of the Earth. UV-C is the most harmful and most energetic of all UV, but is strongly absorbed in both the thermosphere and the stratosphere and does not make it to the Earth’s surface. UV-C is the one responsible for the splitting of oxygen molecules in the stratosphere which leads to the formation of ozone. When ozone absorbs UV it regenerates oxygen atoms and releases heat which warms the upper part of the stratosphere. Since UV-C does not make it to the Earth’s surface, the most harmful form of UV radiation that reaches the surface is UV-B. However, the amount of UV-B that reaches Earth’s surface is significantly reduced because most of it is absorbed by ozone in the stratosphere. Ozone is the only known gas that absorbs UV-B.
Natural conditions in the stratosphere sustain a dynamic balance between the creation and destruction of ozone which helps to ensure the continued existence of the ozone layer. Any disruption of this balance that results in a higher rate of ozone destruction than ozone creation would result in the depletion of ozone. Ozone depletion, consequently, leads to a significant increase in the amount of harmful UV-B radiation that reaches the Earth’s surface. This is what we are talking about when we discuss the ozone problem.
The Ozone Hole
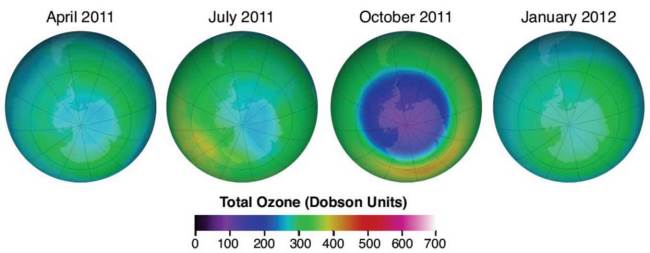
The Ozone Hole is not really a “hole” but rather an area where the thickness of the ozone layer is greatly reduced. This hole is a result of ozone depletion that occurs every year during the Antarctic spring (Figure 6.4) and was first reported to the public by the British Antarctic Survey in 1985. The thickness of the ozone layer above the Antarctic continued to decline while the geographic area covered by the ozone hole continued to increase, reaching its lowest concentration (thickness) in 1994 and largest geographic area in 2000. Recent data shows that ozone concentration globally and in the Arctic and Antarctic is no longer declining.
During the long winter months of darkness over the Antarctic, atmospheric temperatures drop, creating unique conditions for chemical reactions that are not found anywhere else in the atmosphere. During this time, the Antarctic air mass is isolated from the rest of the atmosphere and circulates around the pole in what is known as the polar vortex. This isolation allows temperatures to drop low enough to create ice crystals at high altitudes. Ozone, nitric acid, sulfuric acid and other chlorine-containing molecules are absorbed on the surfaces of these ice particles. When the sun rises over the Antarctic in the southern spring (October), light rapidly releases free chlorine atoms into the stratosphere. The chlorine atoms react with ozone breaking it down to molecular oxygen and an oxygen atom. The polar vortex keeps the ozone-depleted air inside the vortex from mixing with the un-depleted air outside the vortex, hence the formation of an ozone hole.
Ozone Depletion
Global ozone concentrations change periodically with regular natural cycles such as changing seasons, winds, and long-timescale sun variations. Concentrations of ozone in the atmosphere are measured in parts per billion (ppb). Scientists have been measuring ozone since the 1920s using ground-based instruments that look skyward. Satellite measurements of concentrations of atmospheric ozone began in 1970 and continue today.
Ozone depletion occurs when the rate at which ozone is broken down is greater than the rate of its creation, interfering with the dynamic balance between creation and destruction that maintains the ozone layer. When this happens, the amount of harmful UV-B radiation that reaches the Earth’s surface increases. Ozone depletion was first identified over the Antarctic. Scientists suggested that reactions involving man-made chlorine-containing compounds were responsible for depleting ozone in the stratosphere. This hypothesis was based mostly on the physical and chemical properties of these compounds and knowledge about atmospheric conditions.
Chlorofluorocarbons (CFCs) are man-made compounds made up of chlorine, fluorine, and carbon. These compounds were commonly used as propellants in everyday products such as shaving cream, hair spray, deodorants, paints, and insecticides and as coolants in refrigerators and air conditioners. CFCs are extremely stable molecules and do not react with other chemicals in the lower atmosphere, part of the reason why they were considered a safe choice. Their stability means that they tend to remain in the atmosphere for a very long time. With the constant movement of air in the lower atmosphere, CFCs eventually make their way into the stratosphere. Exposure to ultraviolet radiation in the stratosphere breaks them apart, releasing chlorine atoms. Free chlorine (Cl) atoms then react with ozone molecules, taking one oxygen atom to form chlorine monoxide (ClO) and leaving an oxygen molecule (O2) (Figure 6.5). The ClO reacts with other atoms freeing up the Cl making it available to react with another ozone molecule, repeating the cycle over and over, resulting in ozone depletion.
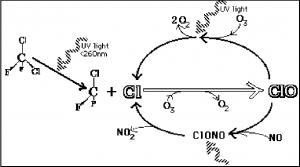
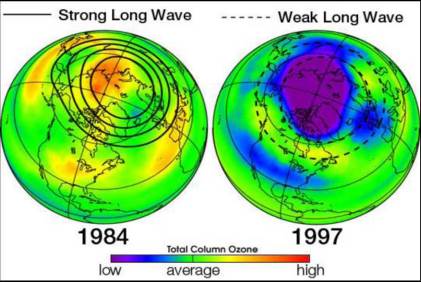
If each chlorine atom released from a CFC molecule destroyed only one ozone molecule, CFCs would pose very little threat to the ozone layer. However, when a chlorine monoxide molecule encounters a free atom of oxygen, the oxygen atom breaks up the chlorine monoxide, stealing the oxygen atom and releasing the chlorine atom back into the stratosphere to destroy another ozone molecule. These two reactions happen over and over again, so that a single atom of chlorine, acting as a catalyst, destroys many molecules (about 100,000) of ozone. The consequence of stratospheric ozone depletion is increased levels of UV-B radiation reaching the Earth’s surface, posing a threat to human health and the environment. Figure 6.6 shows a lower-than-average amount of stratospheric ozone over North America in 1997 when it was abnormally cold compared to 1984, which was warmer than average, showing that ozone depletion does not exclusively affect just the South Pole (Antarctic).
The Montreal Protocol
International policy efforts to restrict the production of ozone-depleting CFCs culminated in the 1987 treaty known as the Montreal Protocol in which signing nations agreed to cut CFC production in half by 1998. At least five follow-up agreements since then helped to deepen the cuts, advanced timetables for compliance, and addressed additional ozone-depleting substances such as halons, methyl chloroform, carbon tetrachloride, and hydrochlorofluorocarbons (HCFCs). Most countries around the world have phased out production of the substances covered by the agreements and industry has been able to shift to safer alternative chemicals. As a result, there’s evidence that the Antarctic ozone hole has stopped growing worse, although recovery is not expected anytime soon. Phasing out CFCs and HCFCs is also beneficial in protecting the earth’s climate, as these substances are also very damaging greenhouse gases.
As part of the United States’ commitment to implementing the Montreal Protocol, the U.S. Congress amended the Clean Air Act (section 6.7), adding provisions for the protection of the ozone layer. Most importantly, the amended Act required a gradual end to the production of chemicals that deplete the ozone layer. The Clean Air Act amendments passed by Congress requires the Environmental Protection Agency (EPA) to develop and implement regulations for the responsible management of ozone-depleting substances in the United States. Under the Clean Air Act, EPA has created several regulatory programs to address numerous issues, including:
• ending the production of ozone-depleting substances,
• ensuring that refrigerants and halon fire extinguishing agents are recycled properly,
• identifying safe and effective alternatives to ozone-depleting substances,
• banning the release of ozone-depleting refrigerants during the service, maintenance, and disposal of air conditioners and other refrigeration equipment,
• requiring that manufacturers label products either containing or made with the most harmful ozone-depleting substances.
Attribution
Zehnder, Caralyn; Manoylov, Kalina; Mutiti, Samuel; Mutiti, Christine; VandeVoort, Allison; and Bennett, Donna, “Introduction to Environmental Science: 2nd Edition” (2018). Biological Sciences Open Textbooks. 4.
https://oer.galileo.usg.edu/biology-textbooks/4
This work is licensed under a Creative Commons Attribution-Noncommercial-Share Alike 4.0 License.