1 Medical Device Innovation and Innovation Process
William Durfee

innovation
Ideas that add value to society
Defining Innovation
Innovation is defined by the introduction of something new, but is also about adding value. Innovative products are new, useful and feasible. All three are needed for innovation. As you are thinking about your new idea, ask yourself, “Is my idea new?, “Is my idea useful, and if so, who will use it and for what purpose?,” and “Is my idea feasible and if so, how will it be engineered and how will it be marketed?”
A theme that runs through this book is that the utility and value of the invention must be determined by the customer, not the inventor. This is a natural result of following a good innovation process, which starts with a problem to be solved where that problem is one that is experienced by the customer. This is particularly important for medical device innovation where new technology, no matter how amazing, that doesn’t solve a significant clinical unmet need is unlikely to succeed.
The Medical Device Innovation Process
There are many new product development processes described in text books and company procedure documents. Most have steps that first involve a front-end of opportunity identification followed by concept development, and then move into steps of detailed product design and implementation followed by product launch and production ramp-up. For example, some companies use a stage-gate process. [1] In some fast-paced industries (not medical), a company will rush a new product to launch without doing extensive preliminaries and then let the market decide success through sales. Consumer electronics and phone apps fall into this category. (For an overview of thinking on product development process, see Katz (2011).[2])
Medical device product development has additional characteristics that can dominate the process. Medical devices must pass a regulatory hurdle. To achieve a CE mark to market your product in Europe, or FDA approval to market your device in the U.S., you must prove that your device is safe and effective. If your device is completely new and comes with a level of risk to the patient, lengthy and costly clinical trials will likely be needed to obtain regulatory approval. Even if your device passes the regulatory hurdle, you must prove to reimbursement agents (medical insurers and government medical agencies) that your device lowers the procedure cost and improves patient outcomes sufficiently to warrant reimbursement. Without reimbursement, your device will not be successful because few patients can afford a large out-of-pocket expense for a medical procedure.
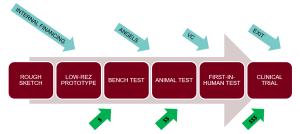
The diagram at the beginning of this section illustrates the main stages of how a typical medical device is developed. The opportunity identification and the initial concept often comes from a clinician who is intimately familiar with a clinical problem that needs solving, and might have a rough concept sketch on a napkin. As the design is refined, renderings and animations are developed to communicate the concept to customers and investors. Low-resolution physical prototypes are created to work out some of the details, followed by initial bench testing to study engineering performance. All of these steps are low cost and typically financed internally, or if at a university, through grant funding.
Next comes testing in animals, first-in-human testing and clinical trials, followed by regulatory approval, reimbursement decisions and product launch. Each step is progressively more expensive and financing becomes a major consideration. For example, for a start-up with an FDA Class III (life-supporting) device, it costs about $150M to take the company through the FDA PMA process.
Another view of the process is shown in the following med-tech innovation figure, which has innovation split into five broad tasks, which can be further reduced to Identify, Invent, Implement stages as popularized by the process described in the Stanford Biodesign text. [3]
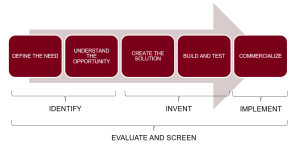
Later chapters in this book cover the innovation steps in detail, but here is a snapshot:
- Define the need State, with some specificity, the customer need, where ‘customer’ could mean physician, nurse, patient, caregiver or other stakeholders. A good medical device innovation process always begins with an unmet need and is needs-driven as the process proceeds.
- Understand the opportunity Become knowledgeable about the disease state, the procedures currently used, the healthcare providers who participate and the problems and opportunities for improvement.
- Create solutions This is the inventive step where multiple concepts that solve the problem are created.
- Assess the solutions The screening process where solutions are assessed to determine if they meet the need, if they are technically feasible and if they are economically viable. The result is down-selecting to one or two concepts to carry forward.
- Further screens In addition to the screens in the step above, one must assess whether the concept can be patented, what the competition is doing, what it will take for the concept to be approved by regulatory agencies, what it will take for the concept to be reimbursed by third-party payers and what business model makes the most sense.
- Building and testing prototype This is where the concept is realized as a physical prototype and bench tested to determine if it works. Later, pre-clinical animal testing on a more realistic prototype is used to refine the concept. If the device is intended to work external to the body, then animal testing is not needed and the process goes straight to first-in-human testing.
- Commercialize Where the process goes from here is variable and depends on the concept and on the environment of the inventor. For example, the inventor could share their idea with an established medical devices company. Or, the inventor may be part of a medical device company already. Or, the inventor could decide to become or collaborate with an entrepreneur and start a new company based on the invention.
The key is to follow a needs-driven innovation process. In other words, first uncover the need, then invent to satisfy the need.
Finding the Significant Opportunity Areas

Value-based healthcare, the idea of evidence-based, better outcomes at lower cost, is now the dominant model for strategics and investors to determine which opportunities to invest in. For example while transcatheter aortic replacement valves have a higher device cost than a surgery replacement valve, the overall cost of the treatment is less because a catheter procedure is faster and cheaper than open-heart surgery.
Likewise, healthcare trends are to shift procedures from hospitals to clinics. For example, free-standing cath labs are trending as they are a lower-cost way to perform less-complicated cardiac procedures in healthier patients than a hospital-based procedure. Another trend is shifting treatment from clinics to the home, and the explosion in wearable sensors and home-based healthcare monitoring, all part of modern digital healthcare, is evidence of the shift to providing the same or better outcomes at an even lower cost.
Further evidence of the dominance of value-based healthcare is that reimbursement is the new bar for medical device success. Reimbursement requires evidence to support claims that a technology leads to better patient outcomes at lower cost than existing solutions, and highlights the importance that you must be able to provide clinical evidence that your device works to its intended purpose, and clinical evidence that better patient outcomes result from using your technology.
Why Innovation is Needed
The reason why medical technology innovation is needed is that it is only through innovation that we can reach the competing goals of better outcomes at lower cost. The new environment is ripe for innovation. Digital healthcare with wearable devices and cloud-based data will drive care providing away from expensive clinical centers. Personalized healthcare will result from sensitive diagnostics and will lead to better outcomes. Big data is being mined for information to produce data-based effectiveness and cost metrics.
At the same time, the major disease drivers will not change. Chronic diseases and conditions such as diabetes, heart disease, cancer, obesity, stroke and arthritis affect six in ten Americans. Heart disease and stroke cost $216 billion each year and arthritis and related conditions cost $140 billion.[4] Devices that address any of these conditions while meeting the new metrics present enormous opportunities for the innovator.
Becoming a Medical Device Innovator
Clinician-Innovators
Innovative ideas can come from expert users who are most familiar with problems that need to be solved. This is particularly true for medical devices where clinicians and other health care providers conceive, by far, the largest number of ideas for new products to solve their problems. For example, a spinal cord injury rehabilitation doctor was acutely aware of the need for patients confined to their beds because of gluteal pressure ulcers, invented and followed through with the development of a prone cart with wheel-chair-like wheels so that patients could roll themselves around the hospital while maintaining their required prone position.
At the same time, creative healthcare professionals generally have little idea of what to do with their idea. They know their invention will solve a problem, but have no experience or training in what it takes to objectively evaluate their idea or how to move it through the commercialization process. Further they rarely have the time to move it through the process. However, as Dr. Chaim Lotan, Chief Innovation Officer, Hadassah- Hebrew University Medical Center, says, “Innovation is a discipline that can be learned!” Further, part of that learning is collaborating with others, particularly engineering experts, to move the idea ahead.
Engineer-Innovators
Other ideas can come from engineers who are starting with a needs-driven process and see solutions for needs they have observed in the clinic. Here it is essential, even in the early stages for the engineer innovator to partner with a clinical champion and together move the idea ahead.
Using This Book
An objective of this book is to help clinicians and engineers to bridge the gap from being simply an inventor to becoming an innovator, someone who can take an invention through the commercialization process. You may have identified the need and perhaps have a solution. What’s next? Knowledge of the innovation process plus capitalizing on the advice and assistance of experts is how your medical technology invention becomes an innovation. This book walks you through the process and you will learn what it takes to de-risk your idea and get it noticed. The book is for clinicians with an idea, academic faculty researchers, leaders of an early stage startup company, intrapreneurs looking to innovate within an established company, medical device innovation fellows who are spending a year in an immersive experience in a medical center to create solutions to unmet needs, and student teams who might be taking an entrepreneurial course or a capstone design course.
The book covers the front end medical device innovation process including the important screens needed to evaluate your idea. It will get you in shape for selling or licensing your idea to an established company or perhaps to launch a startup company of your own. It is not about how to run a startup or how to raise Series B funds or how setup and run a manufacturing line or how to build sales. All of that comes further down the process. While medical technology innovation is never easy, it is still a great time to be a medical technology innovator.
- stage-gate.com ↵
- G. Katz, Rethinking the Product Development Funnel, PDMA Visions, July 2011 (link) ↵
- Zenios, Makower, Yock, Brinton, Kumar, Watkins, Denend, Krummel (2015). Biodesign: the process of innovating medical technologies, 2nd ed. Cambridge University Press. ↵
- cdc.gov/chronicdisease ↵

