15 Preclinical Testing
Paul Iaizzo

Early prototypes of a medical device are generally far from ready to be tested in humans, and must be refined through pre-clinical bench testing and acute and chronic testing in an appropriate animal model. Bench testing and acute animal model testing can be used to iterate on the design. Once you get to chronic testing in animals, the prototype should be under a design freeze.
Bench Testing
Bench testing is often used as lower-cost methods to validate the engineering functions and features of your device before committing to costlier animal and then even much more expensive human studies. Yet, if it a truly novel device that you need to test, you may have to come up with novel, creative, preclinical methodologies. Early bench testing for small products can often be done on a somewhat larger size version: e.g., to understand or emphasize the relative device-tissue interactions. When developing a test plan for the bench studies, the designer must determine the key objectives of the research: even anticipate the desired results and plan the in vitro protocols accordingly.
The typical progression for bench testing is
- Low resolution, low cost models used to screen early prototype ideas.
- More realistic models used to further optimize product designs, understand device-tissue interfaces or to simulate various use states.
- Models built around specific types of patient anatomies: to test and verify the reliability and device functions in a variety of geometries.
- Realistic anatomical and functional models to support the design freeze decision: e.g., testing in reanimated large mammalian organs (e.g., reanimated hearts via the Visible Heart® Methodologies).
- Fresh human cadaver studies with appropriate imaging technologies: i.e., means to determine which imaging modalities are needed.
- High-fidelity models of approved technologies so to support physician training, education, and further feedback needed for next generation technologies.
 High and low-resolution bench testing has become an integral part of the medical device development process from the evaluation of early prototypes to reliability testing, human factors analyses and customer training. The design and complexity of a given bench test is determined by the objectives of the study to be performed, for example, the features or attributes of the product that require critical characterization. In addition, the data needed to be collected defines the methods and materials required for the testing and can help guide how realistic the test needs to be.
High and low-resolution bench testing has become an integral part of the medical device development process from the evaluation of early prototypes to reliability testing, human factors analyses and customer training. The design and complexity of a given bench test is determined by the objectives of the study to be performed, for example, the features or attributes of the product that require critical characterization. In addition, the data needed to be collected defines the methods and materials required for the testing and can help guide how realistic the test needs to be.
Early feasibility testing can sometimes be achieved using basic tissue models, such as chicken breast or beef liver obtained from the supermarket, or delivery system testing in vascular models crafted from silicone tubing from a hardware store. Once a given design has completed the initial screening process, it will undergo a series of optimization steps. Then it is on to accelerated wear testing to determine long-term performance and failure modes, often using custom designed machinery. This kind of bench testing, used to provide the data needed to ensure that the new design is not over or under engineered, does not need to mimic the anatomical environment visually, but is instead built around boundary conditions, measured from either clinical studies, imaging or preclinical testing of predicate devices.
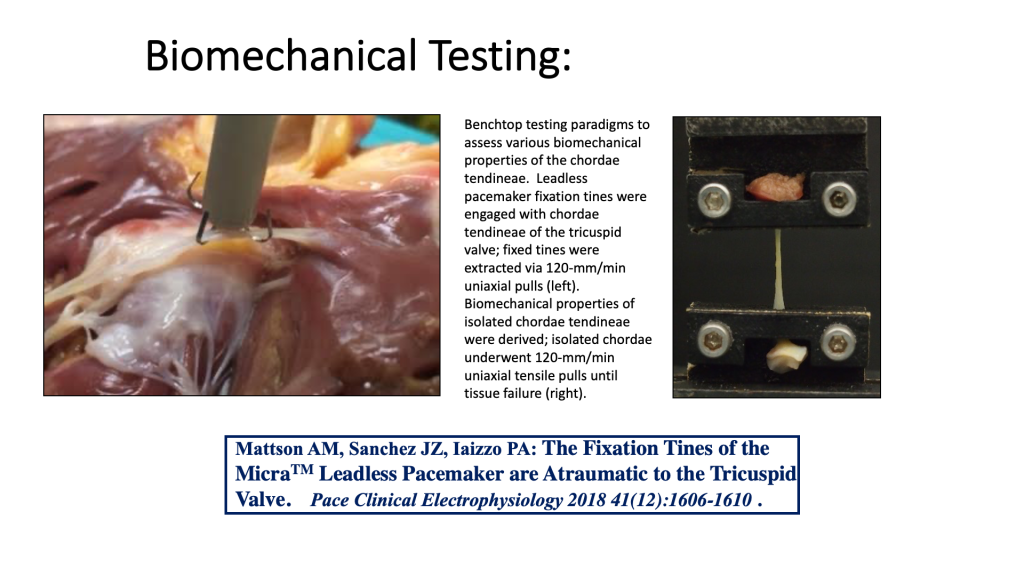
Some pre-clinical bench top models can be quite sophisticated and complex and used for both testing an array of next generation devices, but also are used to collect needed validation datasets for computational modeling.
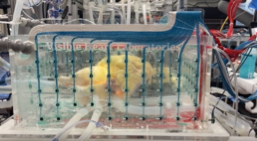
Shown here is a reanimated human heart that was electrically mapped employing modified CardioInsight technologies (Medtronic)
Some Class III medical devices are tested in anatomical models that match the targeted patient disease state, where exceptionally lifelike models can be achieved through advanced imaging of patient anatomy followed by accurate 3D printing of the model. These models can also be used to educate and train physicians prior to the initiation of training in a pre-clinical model or cadaver model.
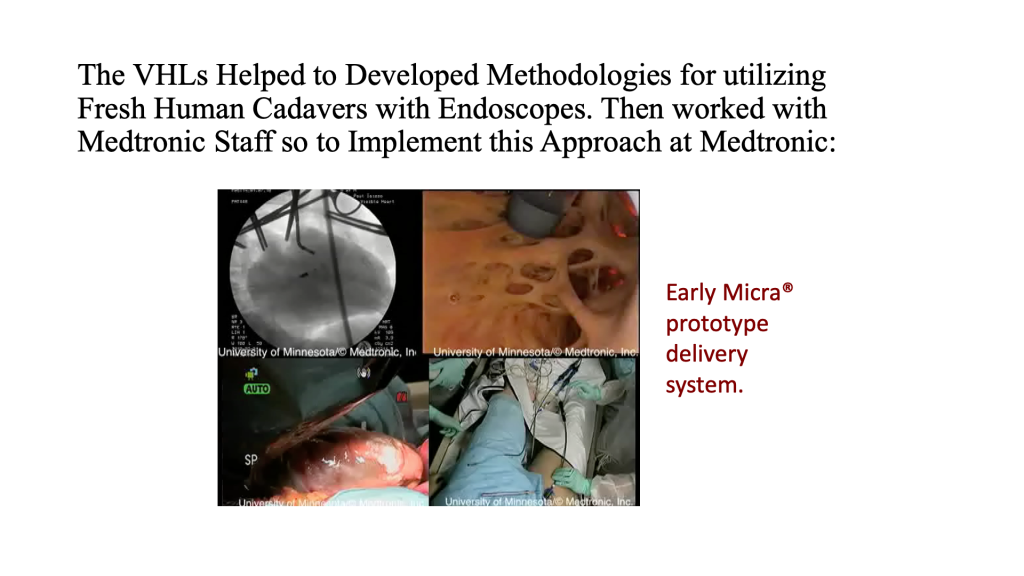
Animal Model Testing
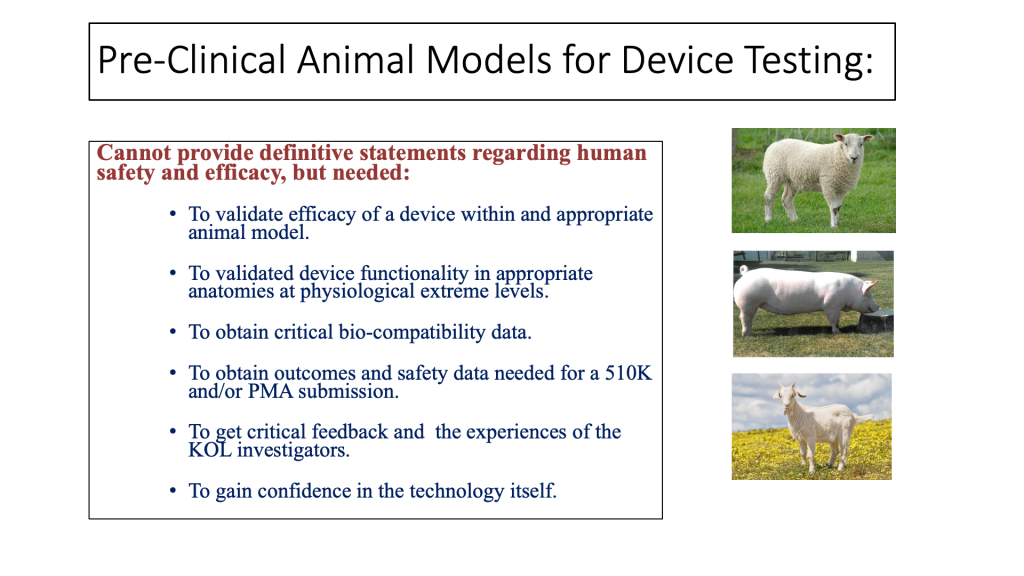
Pre-clinical testing in animals is required for most device submissions to the FDA where there are no predicate devices or associated prior art. For other submissions, some form of animal model testing is likely to be required or desired. It is important that you engage early and often with the FDA or other regulatory agencies as you develop your pre-clinical animal models for testing so that the data you submit matches what the given regulatory body is expecting for an efficient device review.
One of the main goals of pre-clinical animal research is to de-risk the project before moving to clinical testing in humans. While bench tests can indicate whether the engineering performance of a device meets target specifications, only carefully performed animal testing can reveal whether the device elicits the anticipated biological and physiological effects. Yet, the main limitations of any type of animal testing is that it cannot provide a definitive statement regarding the safety and efficacy of the technologies to be used in humans. Because animal testing is expensive and time consuming, you should do everything you can to use bench tests for initial device validation and defining an appropriate design freeze.
In planning an animal study, first identify the risk areas for the product. This might be materials, principles of operation, long term performance/endurance, the placement/delivery procedures or other factors. Then design your test plan to validate each identified risk. Because animal model testing results will be submitted to regulatory agencies as evidence of safety and efficacy, be thinking of specific regulatory guidelines from the start, including the requirements for good laboratory practices (GLP) as defined by U.S. federal law. In addition, there may be standards that dictate best practice testing. For example, cardiac valve testing is covered by ISO 5840. Another consideration is whether the study is acute to determine feasibility or device handling, or chronic to determine long-term performance. Nevertheless, such pre-clinical research is essential to gain confidence in your developed technologies.
Choosing the Animal Model
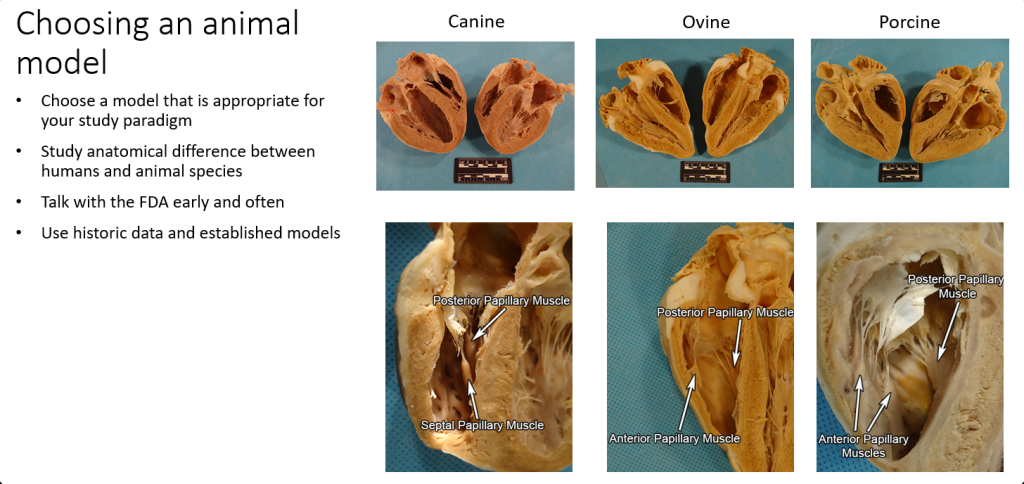
Choosing the appropriate animal model is critical for any successful preclinical study. In developing a pre-clinical study protocol, the device designer must acknowledge that there are both obvious and subtle differences between animal and human anatomies and physiologies. For example, to compare cardiac anatomies across species, it is suggested to use the Atlas of Human Cardiac Anatomy Comparative Anatomy Tutorial [1]. Many specific heart anatomies differs between animals and humans. Trabeculation in swine ventricles are minimal compared to those in the average human, which means a swine model will have limitations if the purpose is to test fixation of a pacing lead within trabeculations. Often there are established standards for the model. For example, stent testing generally uses a porcine coronary model.
If the purpose of the early pre-clinical animal testing is for proof of concept or if the device is in an entirely new category, then innovative animal models can be used. If the purpose is to provide data to the FDA and is part of GLP testing, then standardized models should be used. 
The table shows animal models commonly used for testing cardiac and other types of medical devices.
| Species | Cardiac | Biomaterials Testing | Other Devices |
|---|---|---|---|
| Swine (pig) | Valves, pacemakers, accelerated growth model, valve and pericardial tissues for implants | Preconditioning | Wound healing, pancreas studies, novel MRI methods |
| Caprinae (goat) | LAA closure devices, transcatheter valves | Cervical vertebrae, myotonia | |
| Yucatan (mini-pig) | Long-term implant studies | Tissue engineering | |
| Ovine (sheep) | Chronic valve studies | Tissue engineering | |
| Bovine (cow) | LVAD, artificial heart | Viral studies | |
| Nonhuman primate | Deep brain stimulators, insulin pumps | ||
| Mice, rats | Biocompatibility | Orthopedic devices, neural devices, metabolic disorders |
Pre-clinical studies can be performed on intact animals or in isolated organs.
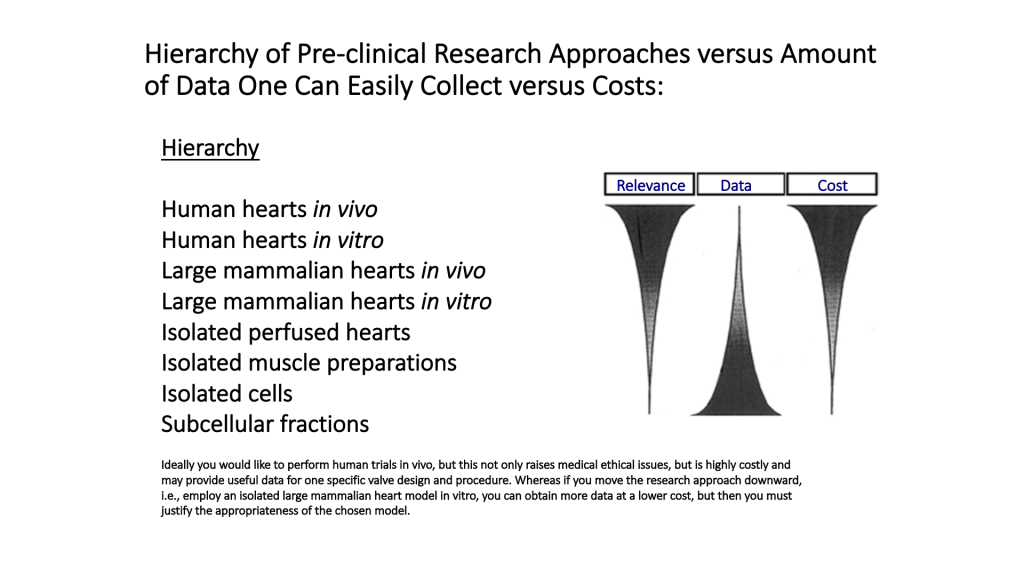
The lower numbered models on the list (1-4) can provide highly relevant data, but come with high costs and fewer available samples. The models later in the list are the opposite: lots of data can be obtained at lower costs, but relevance is reduced.
Animal growth rates also vary dramatically and this can be used as an advantage or become a disadvantage. For example, swine can gain 5-10 pounds per week and in months can be difficult to manage as a research animal or then can be seen as an accelerated growth rate model. Also, it is difficult to observe natural occurring disease states in most large animal models, but progress is being made on transgenic models for such.
IACUC Approval and Oversight
The local Institutional Animal Care and Use Committee (IACUC) must review and approve your protocols prior to conducting any studies in live animals. Most universities that perform pre-clinical animal testing have an IACUC office that will facilitate this process. Large medical technology companies will also maintain an internal IACUC. For startups, it makes the most sense to partner with a university or contract research organization (CRO) to conduct an animal study. By regulation, the IACUC members who review study protocols must include a veterinarian, those with a scientific background that understand the science of the research and a lay person who is unfamiliar with the research. If you are at a university, when submitting a sponsored research proposal, you may not need IACUC approval of your study prior to submission, but will need it before an award is made.
Summary
Testing your idea on the bench and developing preclinical protocols to develop your idea is critical. Although bench and animal testing cannot completely predict device use or its long-term safety and efficacy, you should use these tools to first convince yourself that your device idea is feasible, and then continue your testing to convince other stakeholders including the FDA and the key opinion leaders in your field.
- www.vhlab.umn.edu/atlas/comparative-anatomy-tutorial ↵

