3 Vaccines and Vaccinations: Individual Animals
Learning Objectives
- Describe adverse effects of vaccines and vaccination in dogs, cats, and horses
- Describe cause and clinical manifestations of conditions for which we vaccinate dogs, cats, and horses
- Define what is meant core and non-core (risk-based), in regards to vaccinations
- List core and non-core (risk-based) vaccinations in dogs, cats, and horses
- Create appropriate vaccine protocols for dogs, cats, and horses
This chapter is largely taken from a book for pet owners (Root Kustritz MV (ed), The University of Minnesota Guide to Dog and Cat Wellness, ASIN: B00GCC0YN8).
*
 Adverse Effects of Vaccination in Dogs and Cats
Adverse Effects of Vaccination in Dogs and Cats
Currently there is much controversy among small animal veterinarians and pet owners regarding how frequently vaccinations should be administered to animals. There is no vaccine that is 100% effective and no vaccine that is 100% safe. Vaccines do not induce the same degree of protection in all animals. Knowing, this, how can we decide whether or not to vaccinate a given animal? Your veterinarian does this by assessing the risk factors for your animal and by considering possible adverse effects.
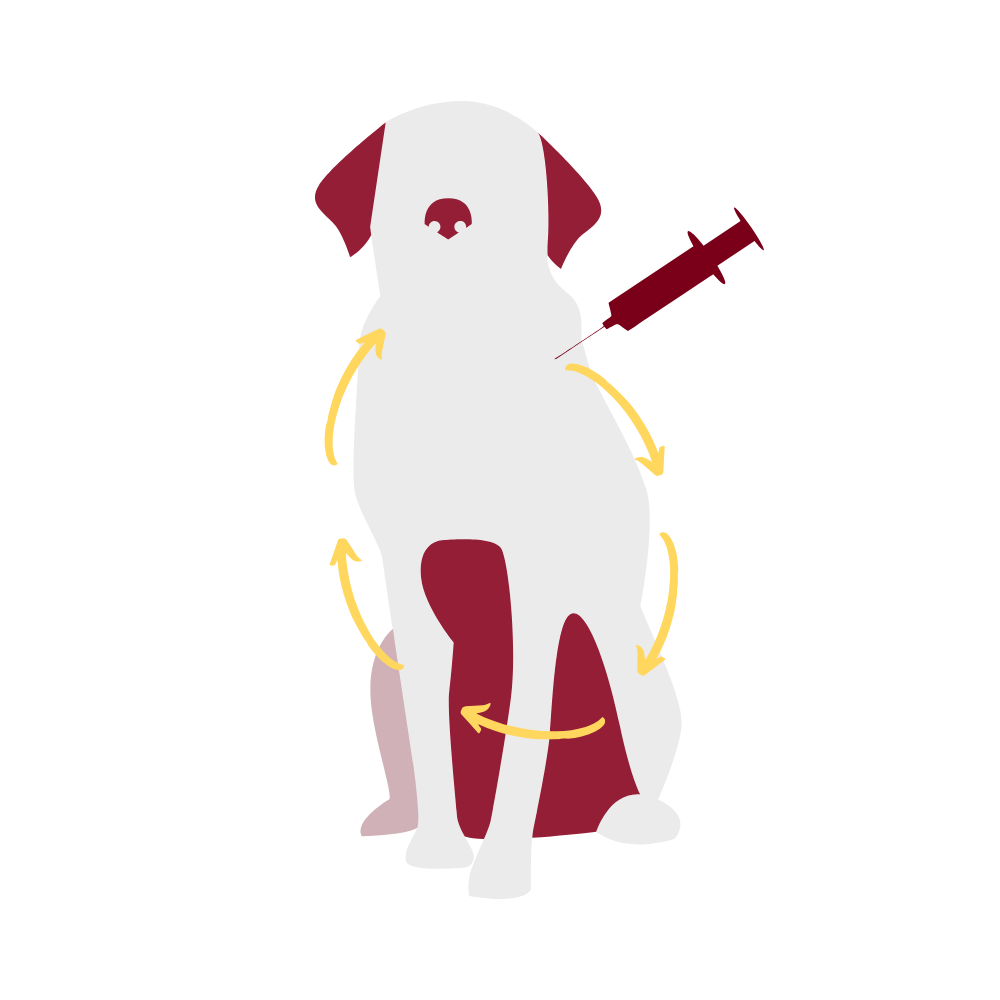
Systemic reactions occur throughout the body, not just at the site of administration of the vaccine. These can be non-specific, causing lack of appetite, fever and soreness that lasts for up to 36 hours after vaccination. This non-specific response is most likely to occur after multiple vaccines are administered and in patients greater than 1 year of age. Allergic reactions may occur. These may be sudden, with onset immediately after vaccination and clinical signs lasting for up to two days. In dogs, 51% of these reactions affect the skin, usually as swellings of the face and ears, and 40% affect the intestinal tract, causing vomiting with or without diarrhea. In cats, 66% of these reactions affect the intestinal tract, 22% affect the respiratory tract, and 12% affect the skin. No particular type of vaccine or manufacturer has been demonstrated to be more likely to cause allergic reactions in dogs and cats. Autoimmune diseases are those conditions in which the immune system of an animal or person starts to destroy that individual’s normal tissue. While people have hypothesized that autoimmune disease could be induced by vaccination, there is no scientific evidence of such a connection in dogs and cats.
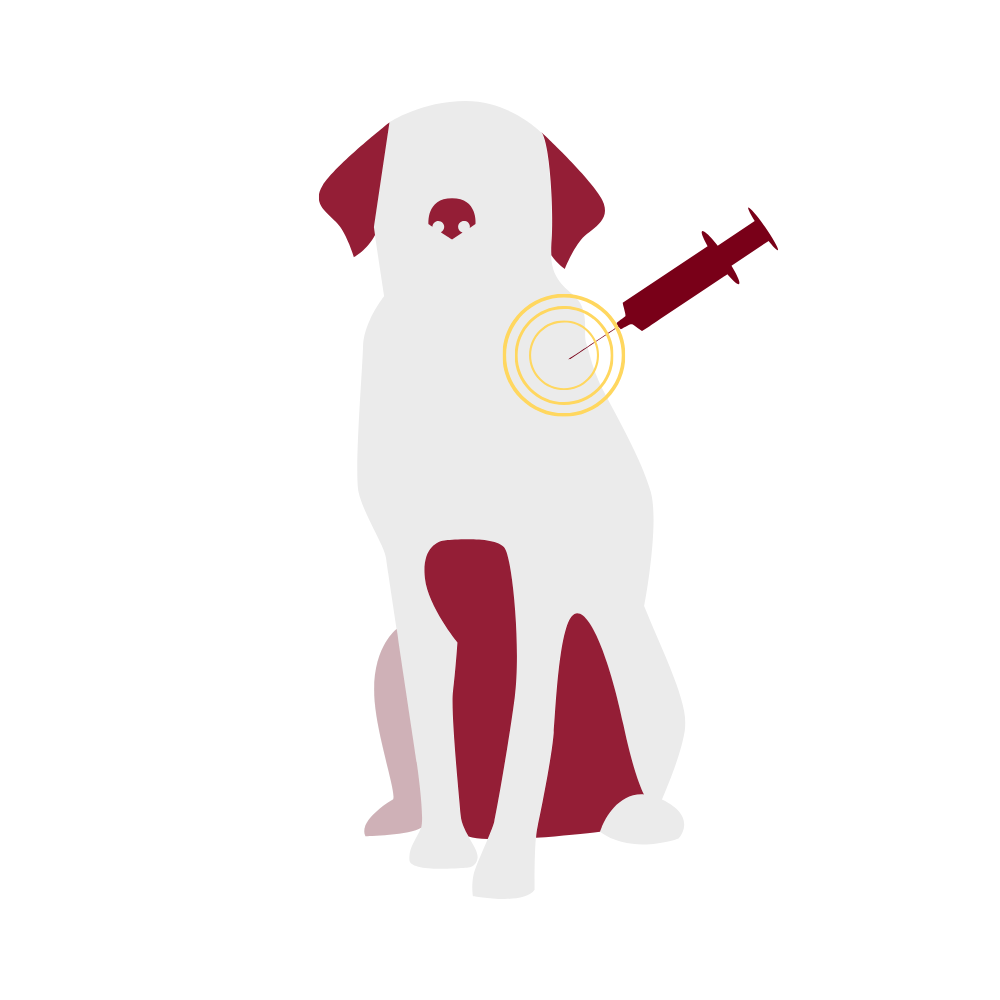
Local adverse vaccine reactions include pain at the injection site, hair loss or change in hair color at the injection site, and swellings. Swellings that develop fairly soon after the vaccination and are soft and non-painful usually are benign sites of inflammation. They resolve over weeks to months and are most commonly seen in dogs after vaccination with rabies vaccine or the distemper combination, and in cats after receiving the rabies vaccination.
Dogs with a history of adverse reactions, especially allergic reactions, may be treated with an antihistamine, a corticosteroid, or both, prior to vaccination. This should not impact how they respond to the vaccine as a single dose of a corticosteroid is not sufficient to cause immunosuppression.
Vaccine-associated feline sarcoma is a malignant tumor that may develop following vaccination in cats. It is reported to occur in 1 in every 1000-10,000 vaccinated cats and is most frequently associated with killed rabies vaccines and vaccines against the feline leukemia virus. Tumors develop weeks to years after vaccination. These tumors are highly invasive, requiring surgical removal and possible chemotherapy. The American Association of Feline Practitioners (AAFP) recommends using non-adjuvanted rabies vaccines for cats in an attempt to decrease the risk of developing vaccine-associated sarcomas. It is unknown at this point if use of non-adjuvanted vaccines decreases the incidence of sarcomas. Because of concerns about development of sarcomas, it is recommended that vaccines not be given in the scruff of the neck, where surgical removal of tumors would be difficult. In North America, vaccines are commonly given in the distal limb, with many practices creating specific practices (“leukemia left, rabies right, rest in the front”, for example, with feline leukemia and rabies vaccines given in the distal left and right rear limbs, respectively, and other vaccines given in the distal forelimbs). Another alternative, described in only one study to date, is vaccination in the tail.
- Use one needle to reconstitute and draw up the reconstituted vaccine, and use a new needle to inject the animal.
- Use the smallest gauge needle possible.
- Deaden the area to be vaccinated using a topical anesthetic cream, such as lidocaine or prilocaine.
- Distract the animal by giving a tasty treat, petting or massaging the animal, catnip, or any other delightful distraction appropriate to the animal and the setting.
- Before vaccinating, dimple or pinch the skin in the area where the needle will puncture the skin.
- Deliver the vaccine slowly.
—“Fear Free”, https://fearfreepets.com/veterinary-professionals/
*
Conditions For Which We Vaccinate Dogs and Cats
| DISEASE | CAUSATIVE ORGANISM | CLINICAL SIGNS | CURABLE? | CONTAGIOUS? |
|---|---|---|---|---|
| CANINE DISTEMPER | Distemper virus | This does not cause a change in the dog’s behavior or temperament. Clinical signs include coughing, sneezing, runny eyes, and neurologic changes. | No specific treatment is available. | Yes, to other dogs and to ferrets |
| INFECTIOUS CANINE HEPATITIS | Adenovirus | Clinical signs are of liver disease with decreased appetite, fever, vomiting, diarrhea, and edema of the cornea, and discharge from the eyes and nose. | No specific treatment is available. | Yes, to other dogs |
| CANINE PARVOVIRUS | Parvovirus | Clinical signs are of severe destruction of the intestinal tract with vomiting and bloody diarrhea. | No specific treatment is available. | Yes, to other dogs and wild canids (wolves) |
| KENNEL COUGH | Bordetella bronchiseptica and parainfluenza virus | Infected dogs have a chronic harsh cough. | Bacterial infection is treatable with antibiotics. | Yes, to other dogs, cats, and pocket pets |
| CANINE INFLUENZA | Canine influenza virus (H3N8, H3N2) | Upper and lower respiratory signs (nasal discharge and cough, respectively), lethargy | No specific treatment is available. | Yes, to other dogs. |
| LEPTOSPIROSIS | Leptospira spp | Clinical signs are those of kidney and liver disease and include fever, lack of appetite, vomiting, and increased thirst. | Yes, with antibiotics. | Yes, through contact with urine from infected animals |
| LYME DISEASE | Borrelia burgdorferei | The most common clinical signs are lameness shifting from leg to leg, and fever. | Yes, with antibiotics. | No, this disease must be transmitted by an infected tick |
| RABIES | Rabies virus | Clinical signs are neurologic; there is a “dumb” form (paralysis) and a “furious” form (aggression, seizures). | No – this disease is invariably fatal. | Yes, to all mammals, including humans |
| FELINE DISTEMPER | Panleukopenia virus | This does not cause a change in the cat’s behavior or temperament. Clinical signs are of severe destruction of the intestinal tract with vomiting and diarrhea. | No specific treatment is available. | Yes, to other cats |
| CALICIVIRUS | Calicivirus | Clinical signs are of severe respiratory disease. | No specific treatment is available. | Yes, to other cats |
| FELINE RHINOTRACHEITIS | Herpesvirus | Rhinotracheitis is inflammation of the nose and respiratory tract, evidenced as discharge from the eyes and nose and sneezing. | No specific treatment is available. | Yes, to other cats |
| FELINE LEUKEMIA | Feline leukemia virus | The immune system is suppressed so chronic infections may be seen. Associated problems are anemia and cancer. | No specific treatment is available. | Yes, to other cats |
- Veterinarians are required to follow the label directions for use of all vaccines
- Frequency of rabies vaccination legally is controlled by municipalities and may be linked to animal licensing
- Puppies and kittens require a series of boosters and revaccination at about 1 year after completion of the initial vaccine series to develop protection against disease
- Vaccines can be designated as “core” or “non-core”
Some vaccination protocols in small animals are being questioned. The vaccine protocols that are being questioned are those for the core vaccines administered to adult dogs and cats. Some data suggest that adult animals may maintain immunity for 5-7 years after their initial vaccinations as young animals, suggesting that the annual revaccination veterinarians had recommended for years is not necessary. Certainly in human medicine, adults are not revaccinated frequently for serious disease but instead in many cases maintain lifelong protection after childhood vaccination. It has been suggested that measurement of antibody concentrations (titers) in animals could be used to determine if vaccination was indicated in a given animal; however, there is confusion regarding how best to measure antibodies in animals and we do not know what concentration of antibody actually is protective against disease. This also is an inaccurate measure of immune status of the animal as it does not account for cell-mediated immunity.
Increased antibody titers have been demonstrated to be well correlated with protection for canine distemper, canine parvovirus, canine adenovirus (hepatitis), and feline parvovirus (panleukopenia). Antibody titers, if high, can be used to support the contention that vaccination has induced immunity. However, if they are low, that does not mean that an anamnestic response cannot be generated by the animal. Examples of patients for whom antibody titer testing may be useful are young animals at the end of their core vaccine series, dogs from breeds with genetic lack of response to parvovirus vaccination (rottweilers, Doberman pinschers, pit bull terriers), dogs with a history of adverse vaccine reactions, dogs with chronic disease, and aged dogs.
Rabies is a special case on all fronts. Frequency of rabies vaccination is mandated by law and that law may not be based in science. Veterinarians do not have the authority to waive the legal requirement for rabies vaccination for dogs with a history of adverse reactions or chronic disease. All rabies vaccines are boostered one year after initial vaccination. Rabies antibody titers are a legal indicator of adequate vaccination and are not considered a legal index of immunity.
The American Animal Hospital Association (AAHA) and AAFP have developed guidelines for vaccination protocols for adult animals.
| VACCINES | DOG | CAT | ||
| CORE | NON-CORE | CORE | NON-CORE | |
| CANINE DISTEMPER VIRUS | X | |||
| CANINE PARVOVIRUS | X | |||
| CANINE ADENOVIRUS-2 (hepatitis) | X | |||
| RABIES | X | X | ||
| FELINE PANLEUKOPENIA VIRUS (distemper) | X | |||
| FELINE HERPESVIRUS-1 | X | |||
| FELINE CALICIVIRUS | X | |||
| FELINE LEUKEMIA VIRUS | X* | |||
| CANINE PARAINFLUENZA VIRUS (kennel cough) | X | |||
| LEPTOSPIRA SP. | X | |||
| BORDETELLA BRONCHISEPTICA (kennel cough) | X | |||
| CANINE INFLUENZA VIRUS | X | |||
| BORRELIA BURGDORFEREI (Lyme disease) | X | |||
| CANINE CORONAVIRUS | X | |||
| CHLAMYDIA (CHLAMYDOPHILA) FELIS | X | |||
| FELINE INFECTIOUS PERITONITIS VIRUS | X | |||
* Considered core for cats less than 2 years of age and for cats that are outside part- or full-time and for cats that live inside but are exposed to outside cats
The University of Minnesota Veterinary Medical Center’s currently recommended vaccination protocols for pediatric and adult dogs and cats are based on the reports and recommendations of the American Veterinary Medical Association (AVMA), AAHA and AAFP. Pros and cons of use of a given vaccine are discussed with the owner, who works in concert with the veterinarian to create the protocol that will be used for their animal. As more research results become available, the recommended vaccines and vaccination intervals may change.
Young animals receive a series of boosters to try to ensure immunization while waiting for maternal antibodies to wane and for the young animal to become immunocompetent. Antibody titers in the bitch can be used as a rough guide to help determine how quickly antibody titers will wane in their offspring; bitches with high titers will have higher concentrations of antibody in their colostrum so pups that receive that colostrum will have transfer of more passive transfer of antibodies and it will take longer for those maternal antibodies to clear, suggesting that those pups will not benefit from very early vaccination. Vaccines should not be given any more frequently than every two weeks, as cytokine release at the time of vaccination may interfere with new vaccines introduced too quickly.
 Preventive Health Care Recommendations for Puppies
Preventive Health Care Recommendations for Puppies
| VISIT NUMBER: AGE | VACCINATIONS GIVEN | PARASITE CONTROL |
| Visit One: 6-8 weeks |
|
|
| Visit Two: 9-12 weeks |
|
|
| Visits Three +/- Four: 14-16 weeks* |
|
|
- The core vaccines (those recommended for all dogs) and parasite control necessary for every puppy are in bold print.
- Vaccinations are boostered every 3-4 weeks until 16 weeks of age. Rabies is given once at 12 weeks of age and again 1 year later.
- *= Current recommendations are for the final distemper combination booster to be given at 16 weeks of age or older.
 Preventive Health Care Recommendations for Kittens
Preventive Health Care Recommendations for Kittens
| VISIT NUMBER: AGE | VACCINATIONS GIVEN | PARASITE CONTROL | TESTS |
| Visit One: 6-8 weeks |
|
|
|
| Visit Two: 9-12 weeks |
|
|
|
| Visits Three +/- Four: 13-16 weeks* |
|
|
- The core vaccines (those recommended for all cats) and parasite control necessary for every kitten are in bold print.
- Vaccinations are boostered every 3-4 weeks until 16 weeks of age. Rabies is given once at 12 weeks of age and again 1 year later.
- *= Current recommendations are for the final distemper combination booster to be given at 16 weeks of age or older.
 Preventive Health Care Recommendations for Adult Dogs
Preventive Health Care Recommendations for Adult Dogs
| EXAMINATION | VACCINES | PARASITE CONTROL | TESTS |
| 1 YEAR OF AGE | |||
|
|
|
|
| 2-6 YEARS OF AGE | |||
|
|
|
|
| GREATER THAN 6 YEARS OF AGE | |||
|
|
|
|
* or at the 1 year anniversary of the last vaccine received as a puppy
- The items in bold print are recommended for every dog. Items not highlighted may or may not be recommended based on the lifestyle of your pet.
- Leptospira vaccine is recommended for all dogs in Minnesota. In general, it is recommended for breeding dogs, duck hunting dogs and farm dogs, or any dog that has routine access to sources of standing water.
- The Lyme vaccine is recommended for dogs that travel in areas where Lyme disease is known to occur commonly, including New England and the upper Midwest. In Minnesota, the area where dogs are most at risk is north of the Twin Cities. Tick prevention is strongly recommended to prevent this disease and other tick-borne illnesses.
- Kennel cough vaccine is recommended for dogs that are exposed to a large number of other dogs, such as dogs that are boarded, show dogs, breeding dogs, and dogs that are housed at “doggy day care” or visit dog parks. Some grooming facilities require this vaccine.
- Frequency of rabies vaccinations legally is mandated by local, not state or federal, law.
- Veterinarians are required to follow the label directions for use of all vaccines.

Preventive Health Care Recommendations for Adult Cats
| EXAMINATION | VACCINES | PARASITE CONTROL | TESTS |
| 1 YEAR OF AGE | |||
|
|
|
|
| 2-7 YEARS OF AGE | |||
|
|
|
|
| GREATER THAN 7 YEARS OF AGE | |||
|
|
|
|
* or at the 1 year anniversary of the last vaccine received as a kitten
- The items in bold print are recommended for every cat. Outdoor or patio cats should receive all of the above vaccinations, parasite control measures, and tests.
- Frequency of rabies vaccinations legally is mandated by local, not state or federal, law.
- Veterinarians are required to follow the label directions for use of all vaccines.
*

 Vaccination of Horses
Vaccination of Horses
Commercial vaccines are available for only a small subset of the infectious diseases to which horses are susceptible. Despite this, vaccination plays an important role in reducing the incidence and severity of common viral and bacterial diseases. There is no such thing as a “one-size-fits-all” vaccination program that is appropriate for use in all horses!
Vaccination programs are tailored to the individual and the farm based on an assessment of:
- Risk of infection and disease (geography, horse age, occupation, movement patterns)
- Medical, economic, and logistic consequences of disease should it occur
- Potential for spread of infection to other horses and/or humans
- Farm size and type
- Vaccine cost, availability, and efficacy
- Risk of adverse reactions to vaccination
Many equine vaccines are available for purchase “over-the-counter”, but horse owners rarely have the knowledge and experience to conduct this type of analysis. Horse owners/managers should therefore make decisions about optimal vaccination programs for each individual horse with the guidance of an equine veterinarian that is familiar with their farm, animals, training/performance goals, and budget. That said, within a given geographical region there is typically a set of core vaccines that are promoted for use in all horses, similar to what was described for small animals. As an example, all horses are highly susceptible to tetanus, a devastating and often fatal disease caused by the bacterium Clostridium tetani. This bacterial organism is widespread in the soil and environment, and readily contaminates the minor wounds and punctures that horses tend to sustain on a frequent basis. Available vaccines are very safe and effective, so are recommended for use in all horses. If you had to identify the single most important core vaccine for horses, it would be tetanus toxoid!
For the remainder of commercial vaccine products, the benefit-risk-cost relationship is less clear and can vary widely between individual horses, different farms, and different geographical regions. These products are categorized as risk-based (non-core) vaccines, which are recommended for some horses and not others. Equine veterinarians use their knowledge and experience to identify horses and farms that may benefit from specific risk-based vaccines. For example, diarrhea caused by rotavirus affects young foals only. Adult horses often carry the virus within their gastrointestinal tracts, but only young foals in the first few months of life develop disease as a result of infection. Some breeding farms suffer high rates of disease caused by this pathogen year after year, while others experience no disease whatsoever. Farms with a significant history of rotavirus enteritis are good candidates for use of the rotavirus vaccine, which is administered to pregnant mares late in gestation to stimulate high levels of anti-rotavirus antibodies in colostrum. These antibodies are consumed and absorbed by the foal in the first day of life, and help to reduce the subsequent risk of infection and disease. There is no reason for adult horses other than broodmares to be vaccinated with this product, nor do all broodmares require such vaccination.
Vaccination is only one element of an infectious disease control program. Horse owners and managers should be aware that farm management practices play just as important a role (for example, sanitation practices, good nutrition and parasite control, controlling horse traffic, quarantine of new arrivals, and quarantine of horses with known contagious disease). They should understand that vaccination does not provide a guarantee of protection at the individual animal level – it lowers the risk of infectious disease but cannot eliminate that risk entirely. Some vaccines are more effective than others, some horses respond to vaccination better than others, many vaccines require multiple doses before protective immunity is achieved, and protective immunity may not be achieved for days to weeks following vaccine administration. Owners who manage their own vaccines should be advised that improper storage, handling, and administration of vaccine products will undermine their effectiveness.
Immune protection of foals is generally achieved through vaccination of the dam in late gestation. Antibodies produced by the mare are concentrated in her colostrum, and then transferred to the foal as it suckles colostrum in the first hours of life. These antibodies provide protection for several months, after which levels gradually decline and the foal must be vaccinated to stimulate its own active immunity. For foals that received adequate amounts of colostrum from a properly vaccinated mare, first vaccines are administered at 4-6 months of age. If they are vaccinated earlier than this, maternal antibody will interfere with an effective immune response to vaccination. By contrast, foals of unvaccinated mares do not have significant concentrations of maternal antibody so vaccination should be initiated earlier. Since it is difficult to predict the precise timeframe during which maternal antibody concentrations wane, foals often receive more doses of vaccine in the initial series compared to adult horses being vaccinated for the first time. For example, a 2-dose initial series is recommended for first-time vaccination of adult horses against tetanus. By contrast, a 3-dose initial series is recommended for foals. This approach helps to ensure that all foals are adequately protected, even those in which maternal antibody may have persisted longer than usual.
The vast majority of equine vaccines are administered by intramuscular (IM) injection, and a few are administered intranasally. Adverse reactions to vaccination are common but in most cases are limited to minor transient muscle swelling and tenderness at the injection site. Occasional horses develop hives, lethargy, fever, or inappetance that may be self-limiting or may require veterinary support. A very small percentage of horses experience serious, and potentially life-threatening, systemic reactions such as anaphylaxis, purpura hemorrhagica, endotoxemia, laminitis, or infection of muscle tissue with the anaerobic bacterium Clostridium sp. (clostridial myonecrosis). Owners should be aware that all vaccines carry an inherent risk of adverse effects, even when the product has been stored, handled, and administered correctly. Administration of vaccines by the veterinarian rather than the owner/manager ensures that an appropriate vaccine product has been chosen; the product has been properly stored, handled, and administered; and that peracute adverse reactions can be treated promptly while the veterinarian is still on the premises.
Serum antibody titers are not widely used to assess an individual horse’s level of immunity to a given infectious disease, or to guide decisions about whether or not vaccination is warranted. Insufficient data are currently available to guide meaningful interpretation of such titers.
*
Core Vaccinations
The AVMA defines core vaccines as those that “protect from diseases that are endemic to a region, those with potential public health significance, required by law, virulent/highly infectious, and/or those posing a risk of severe disease. Core vaccines have clearly demonstrated efficacy and safety, and thus exhibit a high enough level of patient benefit and low enough level of risk to justify their use in the majority of patients.” The following equine vaccines are considered to meet these criteria:
- Tetanus (tetanus toxoid)
- Western Equine Encephalitis (WEE) and Eastern Equine Encephalitis (EEE)
- West Nile Virus
- Rabies
Tetanus
This is a horrifying and often fatal disease caused by the bacterium Clostridium tetani. Spores of the organism are ubiquitous and reside primarily in soil, from where they gain ready access to the body through lacerations, punctures, surgical incisions, and exposed tissue such as the umbilicus of neonatal foals. Bacteria multiply within devitalized tissues at the site of injury or invasion, then produce a neurotoxin that migrates along motor nerves to the spinal cord and central nervous system (CNS). Most mammals are susceptible to this neurotoxin, including humans, but horses are exquisitely sensitive to its effects. Within the CNS, tetanus toxin inhibits the release of inhibitory neurotransmitters, resulting in loss of normal balance and control of muscular contraction. Over 7-10 days, affected horses develop progressive localized and generalized muscle contraction, rigidity, and spasms that are aggravated by stimulation and excitement. Horses often exhibit a characteristic sawhorse stance, raised tail head, prolapse of the third eyelid, and difficulties prehending and masticating feed due to excessive contraction of masticatory muscles (“lockjaw”). They suffer great pain and anxiety related to the muscle spasms and their inability to move or eat normally. Death is often the result of respiratory failure when the muscles of the larynx, intercostal muscles, and diaphragm become affected. Affected horses may be treated with a combination of tetanus antitoxin (antibodies targeting the neurotoxin), penicillin to kill the C. tetani, wound debridement and management to prevent further production of neurotoxin, sedatives, nutritional support, extensive nursing care, and a quiet environment. Whether or not a horse can survive depends upon how early the disease was diagnosed, the speed and severity of disease onset, and how aggressively it can be treated. The good news about tetanus is that it is easily prevented through vaccination! All available vaccines contain tetanus toxoid, a formalin-inactivated form of the tetanus neurotoxin that stimulates a strong humoral immune response following IM administration. The antibodies produced in response to vaccination bind and inactivate the neurotoxin before it can reach the CNS to wreak havoc. Many brands of tetanus toxoid are available and individual products may contain tetanus toxoid alone, or in combination with other antigens as part of a multivalent vaccine. Some products contain all of the core equine vaccines, and others also contain one or more risk-based vaccines. Initial vaccination of a previously unvaccinated adult horse consists of a 2-dose series administered by IM injection 4-6 weeks apart, after which horses are boostered with a single dose annually. Broodmares receive their annual vaccination 4-6 weeks prior to foaling to ensure that high levels of protective antibody may be transferred to the foal via colostrum. Foals then receive a 3-dose series beginning at 4-6 months of age, followed by single annual booster doses as for adults. It is common for horses to receive supplemental boosters if they need surgery or sustain a wound/puncture more than 6 months following their previous vaccination. True protection probably lasts much longer than this, but we err on the side of caution given the horse’s unique susceptibility to this devastating disease.
Western & Eastern Equine Encephalitis
This neurological disease is caused by the related Western (WEE) and Eastern Equine Encephalitis (EEE) alphaviruses, which are endemic in North America. Birds and rodents are the major reservoirs of these viruses, but they may be transmitted to horses and humans by mosquitoes. Following the bite of an infected mosquito, the virus replicates and moves through the bloodstream to the brain, where it causes inflammation and neurological dysfunction. Affected horses exhibit fever, incoordination and weakness, stupor (the common name of the disease is “sleeping sickness”), head-pressing, and terminal seizures and coma. No known antiviral agents are effective in the treatment of this disease, so treatment focuses on supportive care and reduction of CNS inflammation. Fatality rates are 50% for WEE and over 90% for EEE. Horses that survive typically exhibit permanent neurological injury and deficits. The viruses are not directly transmissible from infected horses to other horses or their human handlers, even through mosquitoes, but horses serve as sentinels for increased regional risk of human and equine exposure. The good news about WEE/EEE is that it is easily prevented through vaccination! All available vaccines contain formalin-inactivated WEE and EEE viruses, and they are considered to be very safe and effective in preventing disease. Most vaccines are multivalent products containing WEE/EEE in combination with other core antigens such as tetanus toxoid and West Nile Virus, but versions containing additional antigens are also available. Initial vaccination of a previously unvaccinated adult horse consists of a 2-dose series administered by IM injection 4-6 weeks apart, after which horses are boostered with a single dose annually. Annual boosters are administered in early spring to ensure that horses are well-protected during the summer and fall vector seasons. Broodmares receive their annual vaccination 4-6 weeks prior to foaling to ensure that high levels of protective antibody may be transferred to the foal via colostrum. Foals then receive a 3-dose series beginning at 4-6 months of age, followed by single annual booster doses as for adults. In southeastern states where mosquitoes are active year-round, it is common practice to (1) use a 4-dose primary series for foals beginning at 3 months of age, and to (2) administer boosters every 6 months rather than on an annual basis to ensure that protective immunity is maintained year-round. Because horses are an important part of the surveillance network for arboviruses that also affect humans, WEE/EEE is a reportable disease in Minnesota. When you suspect or confirm this diagnosis you must notify your state veterinary regulatory authorities immediately. In Minnesota, this is the Board of Animal Health.
West Nile Virus Encephalitis
This neurological disease is caused by the West Nile Virus (WNV), a flavivirus for which birds throughout North America are the major reservoir. The virus is transmitted to horses and humans by mosquitoes. After an incubation period of 7-10 days, affected horses develop inflammation of the brain and spinal cord that results in behavior changes, tremors, facial fasciculations, hyperesthesia, weakness, incoordination, and recumbency, seizures, and coma in severe cases. Approximately 30% of cases progress to recumbency and paralysis; these cases carry a poor prognosis and usually are euthanized. No known antiviral agents are effective in the treatment of this disease, so treatment focuses on supportive care and reduction of CNS inflammation. Horses that recover often exhibit neurological deficits that may or may not resolve over the next 6-12 months. The good news about West Nile Virus is that it is easily prevented through vaccination! Four commercial vaccine products are currently available: two contain inactivated virus, one is a live canary pox recombinant vector vaccine, and one is an inactivated flavivirus chimera vaccine. Some versions contain only WNV antigen, while others are multivalent products containing supplemental core or risk-based vaccines. These vaccines are considered to be safe and effective. Specific recommendations vary by product, and the manufacturer’s instructions should be followed. For most of the commercial products, initial vaccination of a previously unvaccinated adult horse consists of a 2-dose series administered by IM injection 4-6 weeks apart, after which horses are boostered with a single dose annually. Annual boosters are administered in early spring to ensure that horses are well-protected during the summer and fall vector seasons. Broodmares receive their annual vaccination 4-6 weeks prior to foaling to ensure that high levels of protective antibody may be transferred to the foal via colostrum. Foals then receive a 3-dose series beginning at 4-6 months of age, followed by single annual booster doses as for adults. In southeastern states where mosquitoes are active year-round, it is common practice to (1) use a 4-dose primary series for foals beginning at 3 months of age, and to (2) administer boosters every 6 months rather than on an annual basis to ensure that protective immunity is maintained year-round. Many veterinarians also use a 6-month booster interval for geriatric and immunocompromised horses. Because horses are an important part of the surveillance network for human disease, WNV is a reportable disease in Minnesota.
Rabies
Rabies is rare in horses, but it is given special consideration because of its public health significance and the fact that disease is almost always fatal. The rabies virus is transmitted in saliva through the bite of an infected wild or domestic animal. The most common vectors vary by geographical region, but in the United States are typically skunks, foxes, raccoons, and bats; in Minnesota, rabies usually is carried by skunks. Horses are typically bitten on the muzzle, face, or lower limbs, but bite wounds may not be visible at the time of diagnosis. Following the bite, the virus migrates along nerves to the spinal cord and brain, where is causes progressive encephalomyelitis that invariably proves fatal. The time period between the bite and onset of neurological symptoms is highly variable, and in other species can range from 1 week to 6 years! Clinical signs of equine rabies are highly variable, but most horses exhibit changes in behavior that are clearly recognized by their owners as uncharacteristic. These may include hyper-alertness, anxiety and mental distress, aggression, hyperactivity such as persistent running, persistent abnormal vocalization, or simply profound depression/somnolence. Affected horses can be extremely dangerous to their owners/handlers as a result of their altered mental status, even in the absence of overtly aggressive behavior. Animals that are not euthanized at this stage will progress to recumbency, paralysis, seizures, coma, and death. The time between first recognition of a problem and death is rarely more than 5-7 days. Definitive diagnosis of rabies requires laboratory testing on the brain. The good news about rabies is that it is easily prevented through vaccination! All commercial vaccines contain killed rabies virus, a potent antigen that triggers strong antibody responses; they are generally considered to be very safe and effective. Initial vaccination of a previously unvaccinated adult horse consists of a single dose of vaccine administered by IM injection, followed by annual booster doses to maintain protective immunity. Broodmares typically receive their annual vaccination 4-6 weeks prior to foaling to ensure that high levels of protective antibody may be transferred to the foal via colostrum, but available evidence regarding duration of immunity indicates that annual vaccination prior to breeding is also acceptable. Foals then receive a 2-dose series (4-6 weeks apart) beginning at 6 months of age, followed by single annual booster doses as for adults. At present, none of the rabies vaccines licensed for use in horses carry a 3-year label as is common for small animal products. Rabies vaccines are available over-the-counter in some states, while other states (including Minnesota) mandate that vaccines be administered by licensed veterinarians only. Because of its zoonotic potential, rabies is a reportable disease of great medical and legal concern.

*
Risk-Based (Non-Core) Vaccinations
Risk-based vaccines are included in the vaccination program on the basis of risk-benefit-cost analysis. Their use varies regionally, between different horse populations within a region, and even between different horses on a single farm. The use of risk-based vaccines should be guided by an equine veterinarian, as horse owners and managers rarely have the knowledge and experience needed for optimum program design. The most commonly used risk-based vaccines in North America include:
- Equine herpesvirus (EHV; rhinopneumonitis or “rhino”)
- Influenza
- Strangles
- Botulism
- Potomac Horse Fever (PHF)
- Equine viral arteritis (EVA)
- Rotavirus diarrhea
- Anthrax
- Leptospirosis
Equine Herpesvirus
Equine herpesviruses 1 and 4 (EHV-1 and EHV-4) are respiratory viruses that cause typical signs of upper respiratory disease: acute fever, lethargy, inappetance, serous nasal discharge, and cough, with full recovery within 2-3 weeks. Clinical disease is most likely to occur in young horses, especially those entering training at facilities in which extensive comingling occurs. EHV-1 is also an important cause of abortion in pregnant broodmares, however, and in rare cases it invades the CNS to cause weakness, incoordination, and inability to urinate and defecate. Epidemics of the neurological form of the disease occur with some regularity, especially among horses assembled at racing or show venues. The virus is spread via direct contact with nasal secretions, aerosolized virus from coughing and sneezing, and in the case of EHV-1 from contact with aborted fetuses, fetal fluids, and the infected placenta. The viruses are ubiquitous and most horses become infected early in life and remain so; the viruses have a complex biology that features a latency state, from which reactivation, viremia, and viral shedding can occur during periods of stress. These factors make infection virtually impossible to control, and explains why outbreaks of EHV can occur in closed populations of horses. Most adult horses develop some immunity as a result of repeated exposure to the viruses; these horses rarely develop significant respiratory disease when infected but can still contribute to spread of the virus within a population. This age- and exposure-related immunity does not protect horses from abortion or neurological involvement, unfortunately. Relegation of EHV vaccines to the risk-based category is the result of this complex virus biology, the high prevalence of latent infection, the fact that most horses develop only mild respiratory disease as a consequence of active infection, and the fact that vaccines are only partially protective. The primary indications for use of EHV vaccines are:
- Prevention of EHV-1 induced abortion in pregnant mares
- Reduction of symptoms and spread of respiratory tract disease (rhinopneumonitis) in foals, weanlings, yearlings, and young performance/show horses that are at particularly high risk of exposure to EHV-1 and EHV-4
EHV-1/EHV-4 vaccines are licensed as aids in prevention of respiratory disease, while specific EHV-1 vaccines are licensed and labeled for prevention of abortion in broodmares. None of the current EHV vaccines are licensed for prevention of herpes myeloencephalitis, the CNS form of EHV-1 infection, nor have any available vaccines been shown to be effective in this regard. All except one vaccine contain formalin-inactivated EHV-1 and EHV-4 (respiratory vaccines) or EHV-1 only (abortion vaccines). Most of these are administered by IM administration only, while one product carries an option for intranasal use once an initial series of IM injections has been administered. Products licensed for prevention of abortion tend to contain larger amounts of viral antigen than those licensed for control of respiratory disease, and these products stimulate stronger immune responses. There is a single modified-live EHV-1 vaccine on the market that is licensed as an aid in preventing respiratory disease due to EHV-1. Initial vaccination of a previously unvaccinated adult horse with a killed virus respiratory vaccine consists of a 3-dose series administered by IM injection 4-6 weeks apart, after which horses are boostered every 6-12 months depending on the stringency of immune protection desired. Horses under 5 years of age, those on breeding farms and in contact with pregnant mares, and performance/show horses are often boostered every 6 months. For abortion prevention, broodmares are vaccinated at 5, 7, and 9 months of gestation using a high-antigen EHV-1 product. This regimen also ensures that colostrum contains significant anti-EHV-1 antibody. Foals then receive a 3-dose series beginning at 4-6 months of age, followed by boosters every 6 months.
Influenza
Influenza is a common viral respiratory disease of horses. As with EHV-1/EHV-4, affected horses exhibit acute fever, lethargy, inappetance, serous nasal discharge, and cough, with full recovery within 2-3 weeks. The virus is spread via direct contact with nasal secretions, and as virus is aerosolized from coughing and sneezing. As with EHV, disease is most likely to occur in young horses, especially those in training at facilities in which extensive comingling occurs (training/show barns, racetracks). The virus does not circulate constantly within a population the way herpesviruses do, but triggers disease when an infected horse is introduced into a naïve population. Vaccination and 14-day quarantine of new arrivals is therefore more effective for control of influenza than for rhinopneumonitis. Since the influenza virus tends to change its genetic and structural makeup over time, vaccines tend to contain multiple strains of virus and are updated on a regular basis to ensure that horses remain protected against viruses in current circulation. Horses under 6 years of age, show/performance horses, and horses boarded at stables with a high level of horse traffic are good candidates for vaccination against influenza. Older horses maintained in a closed herd are at comparatively low risk of infection and disease. There are 3 types of influenza vaccine on the market at present, most of which elicit at least 6 months of protective immunity:
- Many inactivated virus vaccines (IM administration, but for one product boosters may be administered intranasally). Often included in multivalent vaccines targeting EHV-1/EHV-4 or other infectious diseases
- One live canary pox recombinant vector vaccine (IM administration)
- One modified-live vaccine (intranasal administration)
Each of the vaccine types requires a different initial series. Initial vaccination of a previously unvaccinated adult horse with a killed virus vaccine consists of a 3-dose series administered by IM injection, after which horses are boostered every 6-12 months depending on the age of the horse, expected exposure level, and the stringency of immune protection desired. The canary pox recombinant vaccine requires a 2-dose initial series administered by IM injection 4-6 weeks apart, after which horses are boostered every 6-12 months. The modified-live intranasal vaccine requires only a single primary dose, followed by boosters every 6 or 12 months. Broodmares are vaccinated every 6 months, with one of those doses timed for administration 4-6 weeks prior to foaling. Foals are then vaccinated beginning at approximately 6 months of age; as for adults, the primary protocol varies between vaccines but subsequent boosters are administered every 6 months.
Strangles
Strangles is a common and highly contagious respiratory disease of horses caused by the bacterium Streptococcus equi equi. Young horses are most susceptible but horses of any age can be affected. The organism is transmitted by direct contact with infected nasal secretions or purulent material from ruptured lymph node abscesses, and is easily spread on equipment, tack, feed and water buckets, grooming supplies, and human hands or clothing. Horses with active disease shed the highest levels of bacteria, but clinically normal carriers can also shed bacteria and trigger disease outbreaks. Classical signs of disease include fever, copious purulent nasal discharge, lethargy, inappetance, and progressive enlargement, abscessation, and rupture of submandibular, retropharyngeal, and parotid lymph nodes, with subsequent drainage of purulent material. Most horses make a full recovery within several weeks but occasional horses develop life-threatening complications such as upper airway obstruction, pneumonia, spread of infection beyond the respiratory tract (“bastard strangles”), abdominal abscessation, and purpura hemorrhagica, a dangerous immune-mediated disorder. Unless rigorous biosecurity precautions are taken, strangles can sweep through a large percentage of horses in a barn, interrupting the training and show schedule for weeks or months. Even once affected horses have made a full recovery, they may harbor bacteria in the pharynx and guttural pouch for months afterward, serving as a potential source of infection for additional horses. Routine cases of strangles are not treated with antibiotics because they tend to slow progression and resolution of the disease. Treatment is primarily supportive with anti-inflammatory medications used to combat high fever and to encourage horses to eat and drink. Horses with life-threatening complications do receive antibiotic therapy, and horses with immune-mediated disease require concurrent immunosuppressive therapy with corticosteroids. Vaccination against strangles is recommended on premises where strangles is a persistent problem and for horses considered to be at high risk of exposure. Strangles cannot be completely controlled through vaccination, but it does reduce the incidence and severity of disease. Historical efforts to develop safe and effective strangles vaccines were ineffective due to the biology of the causative organism. Many of the vaccines that have been brought to market over the years have carried a high risk of adverse effects and elicited relatively poor levels of immune protection. At present, available vaccines are of two types: a single modified-live vaccine administered by intranasal inoculation, and subunit vaccines containing purified extracts of the Streptococcus equi equi M-protein virulence factor. The modified-live vaccine is considered to be more effective, but is more difficult to administer and carries a higher risk of adverse effects. Vaccination with either product can trigger development of purpura hemorrhagica. Initial vaccination of previously unvaccinated adult horses with the modified-live intranasal vaccine consists of a 2-dose series administered 3 weeks apart, after which horses are boostered every 6-12 months depending on the expected level of exposure and the stringency of immune protection desired. The subunit vaccine requires a 2- or 3-dose initial series administered 2-4 weeks apart, followed by boosters every 6 months. The intranasal modified-live vaccine is not approved for use in broodmares, so when needed the subunit product is administered 4-6 weeks prior to foaling. Foals then receive a 3-dose series beginning at 4-6 months of age (subunit vaccine) or 6-9 months of age (intranasal vaccine), followed by boosters every 6 months if ongoing risk of exposure is anticipated. Horses that have recovered from strangles develop strong immune responses that provide protection for approximately 5 years. Subsequent vaccination should be done with caution, as vaccination in the face of existing immunity increases the risk of purpura hemorrhagica.
Botulism
This is a serious and life-threatening disease caused by a toxin produced by the bacterium Clostridium botulinum. Bacterial spores gain access to the body either through ingestion or wound contamination, after which the spores vegetate and toxin is produced. Alternatively, preformed botulinum toxin may be consumed when horses eat decaying or improperly preserved hay, haylage, or silage, or feed contaminated with rotting remnants of animal carcasses (a decomposing dead rabbit incorporated during round bale production, for example). Horses are exquisitely sensitive to the biological effects of botulinum toxin, which blocks acetylcholine release at the neuromuscular junction to cause profound flaccid paralysis of muscle. Clinical signs in horses include generalized weakness progressing rapidly to recumbency, tremors, inability to prehend and swallow feed (dysphagia), secondary injury related to falls, and death by respiratory paralysis. There are 8 distinct toxins produced by different subtypes of Cl. botulinum; types B (90% of cases) and C (10% of cases) are responsible for most cases of equine botulism. Type B botulism occurs most often in the Mid-Atlantic states and Kentucky, while Type C botulism is most common in Florida. Affected horses may be treated with a combination of antitoxin (antibodies targeting the neurotoxin), antibiotics to kill the C. botulinum organism, laxatives to encourage passage of any toxin within the gut, nutritional support, extensive nursing care, and mechanical respiratory ventilation for horses in respiratory failure. Prognosis for life depends upon how early the disease was diagnosed, the speed and severity of disease onset, how aggressively it can be treated, and whether the horse remains standing. Horses that survive can take weeks to recover fully. Vaccination is recommended for horses in endemic regions, or horses scheduled for transport to endemic regions. Minnesota horses are not routinely vaccinated against botulism, as the disease is rare here. By contrast, Minnesota broodmares that will be transported to Kentucky in the spring for foaling and breeding are typically vaccinated to optimize protection for the mare and her foal in that environment. Available vaccines contain inactivated Type B botulinum toxin (toxoid). There are no licensed vaccines containing Type C or any other toxin sub-type. Initial vaccination of previously unvaccinated adult horses with botulinum toxoid consists of a 3-dose series administered by IM injection 4 weeks apart, after which horses receive annual boosters. Broodmares typically receive their annual vaccination 4-6 weeks prior to foaling to ensure high levels of antibody in colostrum. Foals then receive a 3-dose series (4 weeks apart) beginning at 2-3 months of age, then boostered at 12 months of age, and annually thereafter. This vaccine may be administered earlier than others because maternal antibody shows little interference with vaccination.
Potomac Horse Fever
Potomac Horse Fever (PHF) is caused by Neorickettsia risticii, a bacterial organism that is transferred to horses when they inadvertently ingest aquatic insects near rivers, streams, ponds, or marshland. Most infections are subclinical, but some horses develop fever, diarrhea, endotoxemia, and laminitis. Severe cases may be fatal or result in euthanasia of the case due to cost of management and/or occurrence of severe laminitis. In Minnesota most cases are seen from July to September. Young foals are less susceptible to this disease than adults. Commercial killed bacterin products are available but efficacy is questionable; clients should not expect prevention of disease in endemic regions. Not only is there little scientific evidence that vaccination induces protective immunity, but vaccines contain a single strain of Neorickettsia risticii while natural disease has been attributed to multiple field strains. Anecdotally, veterinarians in endemic regions believe that vaccination may reduce the incidence and severity of disease. Vaccinations should be timed to precede the peak period of exposure during summer/fall months. PHF is available as both a standalone vaccine and as a combination PHF-rabies vaccine. Initial vaccination of a previously unvaccinated adult horse with the killed bacterin consists of a 2-dose series administered by IM injection 3-4 weeks apart, after which the horse is boostered every 6-12 months depending on the regional level of exposure and risk of disease. Broodmares typically receive their annual vaccination 4-6 weeks prior to foaling. Foals then receive a 2-dose series (3-4 weeks apart) beginning at 5 months of age, and are then boostered at 12 months of age, and every 6-12 months thereafter.
Equine Viral Arteritis
Equine viral arteritis (EVA) is a viral disease of particular significance to the equine breeding industry. Infection is rarely life-threatening, but the virus can establish a long-term carrier state in breeding stallions and cause abortion in pregnant broodmares. The virus is transmitted in respiratory secretions and also in the semen from infected stallions. Most horses that become infected show no signs of disease, but clinical signs that do occur include fever, edema of the lower limbs and ventral abdomen, hives, serous nasal and ocular discharge, and abortion. Infection of intact males can result in a life-long carrier state within the reproductive tract, and these horses are the key reservoirs of infection within the population. A modified-live EVA vaccine is commercially available, and is considered safe and effective in stallions and non-pregnant mares. It is not widely used, but is reserved for use in specific circumstances. Reasons for vaccination include:
- To protect stallions against infection and subsequent development of a carrier state
- To immunize seronegative mares before being bred with known-positive semen
- To stop outbreaks in non-breeding populations
EVA is a reportable disease because of its significance to the breeding industry. When planning a vaccination program against EVA veterinarians should consult with state veterinary officials. Since is not possible to differentiate serological responses to vaccination from those induced by natural infection, horses being vaccinated for the first time are tested first to confirm that they are seronegative. Vaccination status may also influence the ability to export a horse in future; vaccinated horses become seropositive and this can bar them from export to certain countries. Initial vaccination of a previously unvaccinated seronegative adult horse with the modified-live EVA vaccine consists of a single dose administered by IM injection, followed by annual boosters. Broodmares are vaccinated prior to breeding, and stallions are vaccinated approximately 4 weeks prior to the breeding season.
Rotavirus Diarrhea
Rotavirus is a major cause of infectious and contagious diarrhea in young foals. Adults can become infected and shed rotavirus in manure, but do not develop clinical disease. The virus is transmitted by the fecal-oral route and destroys small intestinal villi, leading to maldigestion, malabsorption, and profuse diarrhea. The manure from affected foals contains high levels of virus that cause widespread contamination of barns and paddocks. Fatality rates are low as long as foals receive adequate supportive therapy including fluids, electrolytes, and nutritional support. Although vaccination is a useful aid in reducing the incidence and severity of rotaviral enteritis, biosecurity procedures play an even more important role. An inactivated vaccine containing Group A rotavirus is commercially available, and is used on breeding farms with a history of significant rotaviral disease. This vaccine is not administered to foals, but to pregnant broodmares in late gestation with the goal of eliciting high levels of anti-rotavirus antibody in the colostrum. The vaccine is considered to be safe and effective. Pregnant mares receive a 3-dose series of IM vaccinations at 8, 9, and 10 months of gestation. In order for colostral antibody to protect the foal, the foal must consume an adequate volume of colostrum and absorb the antibody it contains.
Anthrax
This is a rapidly fatal type of septicemia caused by the bacterium Bacillus anthracis. Horses and other species (including humans) become infected through ingestion, inhalation, or wound contamination by bacterial spores within the soil. Survival of spores is enhanced by alkaline soil conditions, so the disease tends to occur only in specific geographical regions, including pockets of North and South Dakota. Vaccination is warranted only for horses that are pastured in endemic regions. A single vaccine product is licensed for use in horses. It contains live non-encapsulated bacterial spores administered by subcutaneous injection. Vaccination of pregnant mares is not recommended. Initial vaccination of a previously unvaccinated adult horse consists of a 2-dose series (2-3 weeks apart) administered by subcutaneous injection, followed by annual boosters. If broodmares are vaccinated, this should be done when they are open (non-pregnant) prior to the breeding season. There is little information to guide vaccination programs in foals, and foals are more likely to experience adverse effects following vaccination compared to adult horses.
Leptospirosis
Leptospirosis is a zoonotic disease, caused by bacteria of the genus Leptospira. There are many different serotypes of disease and prevalence varies with geographic location. Healthy horses may carry multiple serovars and clinical disease occurs sporadically. The serovar most commonly associated with disease in horses is pomona. Infection is acquired through exposure to the organism via the mucous membranes or abraded skin. The leptospiral organisms are shed in the urine of infected horses (additionally the placenta, fetal fluids, and urine of the mare in abortion cases) and a number of wildlife hosts can shed Leptospira spp. in the urine as well. Clinical manifestations of disease include inflammation within the eye (uveitis), late-term abortion, and acute renal failure. There is one vaccine approved for use in horses in the United States. It is a killed vaccine and is labeled for use in healthy horses 6 months of age or older. Duration of immunity of this product has not been determined. Vaccination will prevent animals from shedding leptospiral organisms in urine. Horses 6 months of age or older should receive two initial doses, 3-4 weeks apart with annual revaccination. The product can safely be used in mares up through the second trimester of pregnancy.

*
 Extra Resources
Extra Resources
- AAEP Vaccine guidelines and vaccine charts, adults and foals: https://aaep.org/guidelines/vaccination-guidelines
- 2017 AAHA Vaccine guidelines: https://www.aaha.org/guidelines/canine_vaccination_guidelines.aspx
- 2015 WSAVA Vaccination guidelines for dogs and cats: https://www.wsava.org/guidelines/vaccination-guidelines
- Purpura hemorrhagica: https://ker.com/equinews/purpura-hemorrhagica-horses/
- Laminitis: https://www.merckvetmanual.com/musculoskeletal-system/lameness-in-horses/laminitis-in-horses

