70 Formation of Organic Molecules in an Earthly Reducing Atmosphere
A prerequisite to the prebiotic chemical experimentation is a source of organic molecules. Just as life requires energy (to do anything and everything!), converting inorganic molecules into organic molecules requires an input of free energy. As we have seen, most living things today get free energy by oxidizing nutrients or directly from the sun by photosynthesis. Recall that in fact all the chemical energy sustaining life today ultimately comes from the sun. But, before there were cells, how did organic molecules form from inorganic precursors? Oparin and Haldane hypothesized a reducing atmosphere on the prebiotic earth, rich in inorganic molecules with reducing power (like H2, NH3, CH4, and H2S) as well as CO2 to serve as a carbon source. The predicted physical conditions on this prebiotic earth were:
- lots of water (oceans).
- hot (no free O2).
- lots ionizing (e.g., X, 𝛾) radiation from space, (no protective ozone layer).
- frequent ionizing (electrical) storms generated in an unstable atmosphere.
- volcanic and thermal vent activity.
Origins of Organic Molecules and a Primordial Soup
Oparin suggested that abundant sources of free energy fueled the reductive synthesis of the first organic molecules to create what he called a “primeval soup”. No doubt, he called this primeval concoction a “soup” because it would have been rich in chemical (nutrient) free energy. The Oparin/Haldane proposal received strong support from the experiments of Stanley Miller and Harold Urey (Urey had already won the 1934 Nobel Prize in Chemistry for discovering deuterium). Miller and Urey tested the prediction that, under Haldane and Oparin’s prebiotic earth conditions, inorganic molecules could produce the organic molecules in what came known as the primordial soup. Their famous experiment, in which they provided energy to a mixture of inorganic molecules with reducing power, is illustrated below (Figure 1).
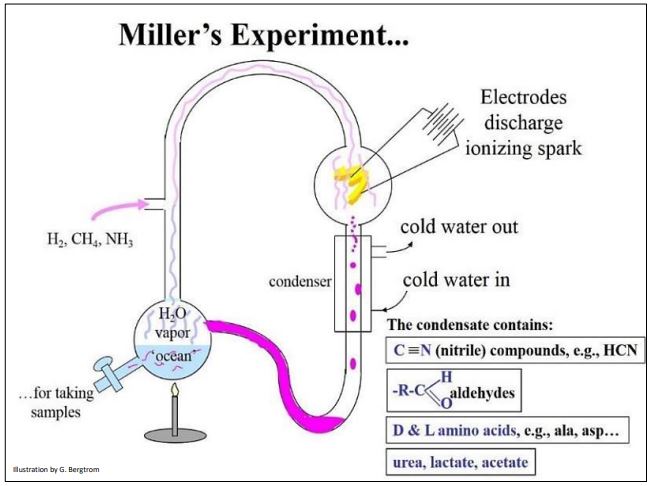
Miller’s earliest published data indicated the presence of several organic molecules in their ocean flask, including a few familiar metabolic organic acids (lactate, acetate, several amino acids…) as well as several highly reactive aldehydes and nitriles. The latter can interact in spontaneous chemical reactions to form organic compounds. Later analyses further revealed purines, carbohydrates and fatty acids in the flask. Later still, 50 years after Miller’s experiments (and a few years after his death), some un-analyzed sample collection tubes from those early experiments were discovered.
When the contents of these tubes were analyzed with newer, more sensitive detection techniques, they were shown to contain additional organic molecules not originally reported, including 23 amino acids. To read more, see Surprise Goodies in the Soup!
Clearly, the thermodynamic and chemical conditions proposed by Oparin and Haldane could support the reductive synthesis of organic molecules. At some point, Oparin and Haldane’s evolving chemistries would have to have been internalized inside of semipermeable aggregates (or boundaries) destined to become cells. Examples of such structures are discussed below. A nutrient-rich primordial soup would likely have favored the genesis of heterotrophic cells that could use environmental nutrients for energy and growth, implying an early evolution of fermentative pathways similar to glycolysis. But, these first cells would quickly consume the nutrients in the soup, quickly ending the earth’s new vitality!
So, one must propose an early evolution of least small populations of cells that could capture free energy from inorganic molecules (chemoautotrophs) or even sunlight (photoautotrophs). As energy-rich organic nutrients in the ‘soup’ declined, autotrophs (notably photoautotrophs that could split water using solar energy) would be selected. Photoautotrophs would fix CO2, reducing it with H– ions from water. Photoautotrophy (photosynthesis) would thus replenish carbohydrates and other nutrients in the oceans and add O2 to the atmosphere.
Oxygen would have been toxic to most cells, but a few already had the ability to survive oxygen. Presumably these spread, evolving into cells that could respire, i.e., use oxygen to burn environmental nutrients. Respiratory metabolism must have followed hard on the heels of the spread of photosynthesis.
Photosynthesis began between 3.5 and 2.5 billion years ago (the Archaean Eon). Eventually, photosynthetic and aerobic cells and organisms achieved a natural balance to become the dominant species in our oxygen-rich world.
The Tidal Pool Scenario for an Origin of Polymers and Replicating Chemistries
In this scenario, prebiotic organic monomers would concentrate in tidal pools in the heat of a primordial day, followed by polymerization by dehydration synthesis. The formation of polymer linkages is an ‘uphill’ reaction requiring free energy. Very high temperatures (the heat of baking) can link monomers by dehydration synthesis in the laboratory, and may have done so in tidal pool sediments to form random polymers. This scenario further assumes that the dispersal of these polymers from the tidal pools with the ebb and flow of high tides. The tidal pool scenario is illustrated below (Figure 2).
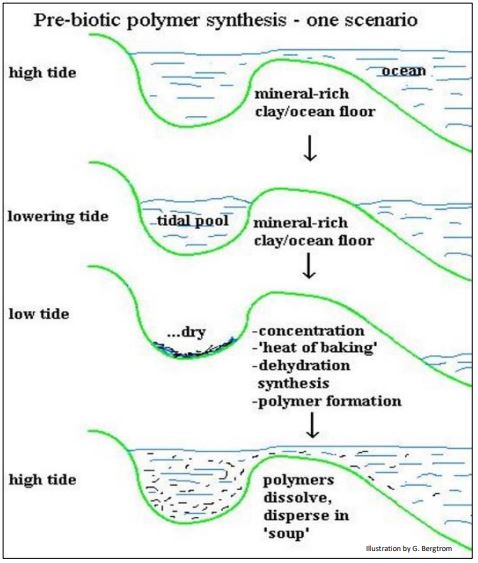
The concentration of putative organic monomers at the bottom of tidal pools may have offered opportunities to catalyze polymerization, even in the absence of very high heat. Many metals (nickel, platinum, silver, even hydrogen) are inorganic catalysts, able to speed up many chemical reactions. The heavier metals were likely to exist in the earth’s crust as well as in the sediments of primordial oceans, as they do today. Such mineral aggregates in soils and clays have been shown to possess catalytic properties. Furthermore, metals (e.g., magnesium, manganese…) are now an integral part of many enzymes, consistent with an origin of biological catalysts in simpler aggregated mineral catalysts in ocean sediments.
Before life, the micro-surfaces of mineral-enriched sediment, if undisturbed, could have been able to catalyze the same or at least similar reactions repeatedly, leading to related sets of polymers. Consider the possibilities for RNA monomers and polymers, based on the assumption that life began in an RNA world. The possibilities are illustrated below in Figure 3.
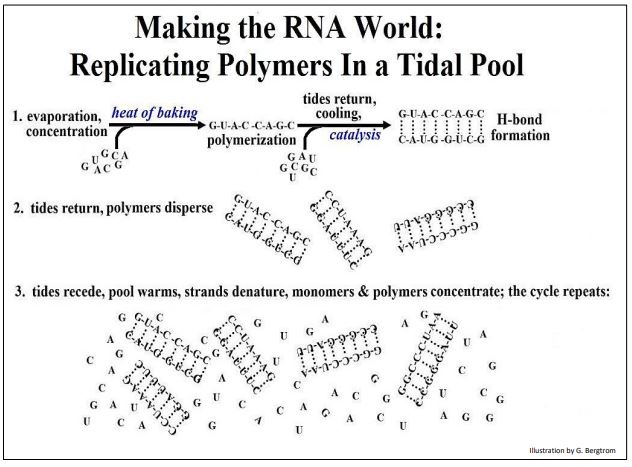
The result predicted here is the formation not only of RNA polymers (perhaps only short ones at first), but of H-bonded double-stranded RNA molecules that might effectively replicate at each cycle of concentration, polymerization and dispersal. Heat and the free energy released by these same reactions could have supported polymerization, while catalysis would have enhanced the fidelity of RNA replication.
Of course, in the tidal pool scenario, repeated high heat or other physical or chemical attack might also degrade newly formed polymers. But what if some RNA double strands were more resistant to destruction. Such early RNA duplexes would accumulate at the expense of the weaker, more susceptible ones. Only the fittest replicated molecules would be selected and persist in the environment! The environmental accumulation of structurally related, replicable and stable polymers reflects a prebiotic chemical homeostasis (one of those properties of life!)
Watch this video to learn about life origins in a reducing atmosphere.
Overall, this scenario hangs together nicely, and has done so for many decades. However, there are now challenging questions about the premise of a prebiotic reducing environment. Newer evidence points to an earth atmosphere that was not at all reducing, casting doubt on the idea that the first cells on the planet were heterotrophs. Recent proposals posit alternative sources of prebiotic free energy and organic molecules that look quite different from those assumed by Oparin, Haldane, Urey and Miller.
This chapter by Gerald Bergtrom is licensed CC BY 4.0.