Light and Eyeballs
81 Physics of Light
Learning Objectives
Being able to describe the basic properties of light.
Know what factors determine the color of an object.
Basic Properties of Light
We define visible light as the electromagnetic energy that interacts with the photoreceptors in our eyes. A honeybee would have a different definition of visible light, because it can see ultraviolet rays (200-400nm); UV is invisible to us, which can make us unaware of sunburn risk!
Electromagnetic radiation can be conceptualized both as a wave and as a particle. In the wave framework, we think of light as a traveling wave, similar to sound waves, but instead of air pressure varying over time, we now have tiny changes in the local electric and magnetic fields. The rate at which the fields rise and fall as a wave travels through them is the wave’s frequency; the distance across space between two peaks of the wave is its wavelength. All electromagnetic radiation travels at the same speed (300 million meters per second in a vacuum), and for traveling waves it turns out that speed = wavelength x frequency. This fact means that light with longer wavelengths has electromagnetic fields that oscillate at lower frequencies.
The electromagnetic spectrum is a way of illustrating many different wavelenths of this radiation. The portion of the electromagnetic spectrum that we call visible light is just a tiny portion of this spectrum, which contains all different kinds electromagnetic waves, that lies between 400 and 700 nanometers. These are the wavelengths of light that are absorbed by the photoreceptors in our eyes.
Electromagnetic radiation can also be conceptualized as moving particles. This is in part because the radiation takes on only specific values of intensity, which we refer to as being quantized. The smallest intensity value corresponds to a single particle, called a photon, which is the smallest amount of light that can be generated or transmitted.
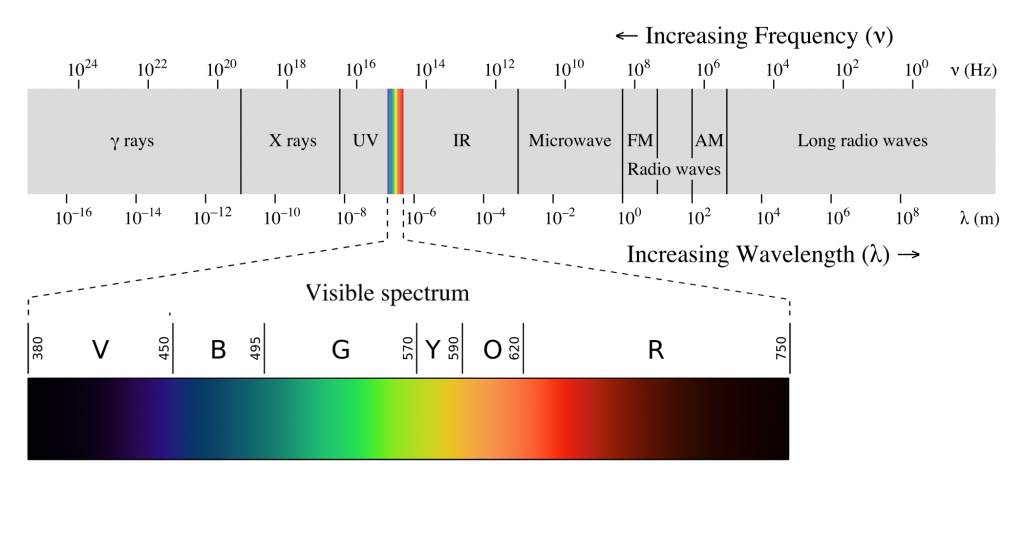 Figure 8.1.1.. Electromagnetic spectrum. Humans are only able to perceive wavelengths of light between 400nm and 700nm. The wavelength is directly related to the perceived color that is seen. Shorter wavelengths are perceived as violet, where are longer wavelengths are perceived as red. Credit: EM_spectrumrevised © Philip Ronan, Gringer is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
Figure 8.1.1.. Electromagnetic spectrum. Humans are only able to perceive wavelengths of light between 400nm and 700nm. The wavelength is directly related to the perceived color that is seen. Shorter wavelengths are perceived as violet, where are longer wavelengths are perceived as red. Credit: EM_spectrumrevised © Philip Ronan, Gringer is licensed under a CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
Factors that Determine Color
Just as pitch is related to the frequency of sound waves, color is related to the wavelength of light. Monochromatic light- defined as containing only one wavelength, when reaching the eye on a black background has roughly the colors shown in the spectrum diagram. However, in most situations, the color of an object is determined by the wavelengths of light that the object reflects and absorbs, as well as the wavelengths of light coming from the surrounding context. Later sections of this book describe how color perception depends on the illumination, the object, and its context.
CC LICENSED CONTENT, SHARED PREVIOUSLY
Cheryl Olman PSY 3031 Detailed Outline
Provided by: University of Minnesota
Download for free at http://vision.psych.umn.edu/users/caolman/courses/PSY3031/
License of original source: CC Attribution 4.0
Adapted by: Nura Ahmed and Victoria Manchanthasouk
Schwiegerling, J. (2004). Field Guide to Visual and Ophthalmic Optics,
SPIE Press, Bellingham WA.
Retrieved from: https://spie.org/publications/fg04_p04_photoreceptors?SSO=1
CC BY-NC-SA
Adapted by: Victoria Manchanthasouk
Quiz by: Morgan Mauch

