Taste and Smell
40 Appetite
Learning Objectives
Be able to describe how orbitofrontal cortex impacts appetite.
Understand why it matters that blood sugar inhibits responses in the nucleus of the solitary tract.
Understand why texture affects appetite.
The control of appetite and food intake can be divided into “homeostatic” and “non-homeostatic” control. The alteration in consumption of food that follows sensing of energy balance forms the basis of the homeostatic control of appetite: following a meal, appetite is suppressed, whereas following significant energy expenditure, we feel hungry. Postprandial satiety signals include changes in circulating concentrations of nutrients and gut hormones.
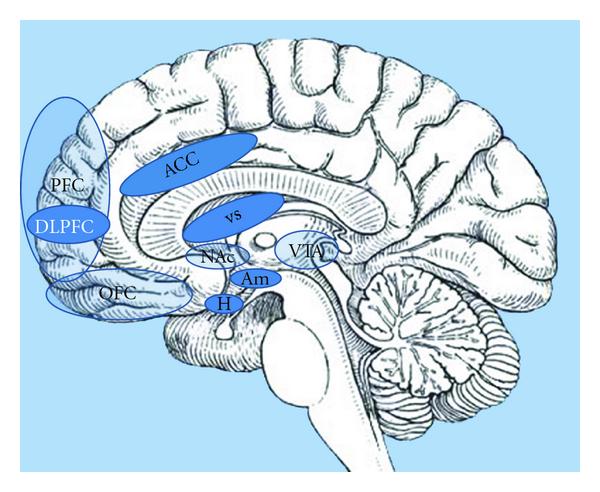
Key areas include the amygdala, hippocampus, insula, striatum, and orbitofrontal cortex (OFC), although this list is by no means exhaustive. It is now widely accepted that, with regard to appetite control, homeostatic and non-homeostatic systems do not function independently; instead, there is extensive cross-modulation between them, along with a complex integration of inputs before a final decision is made regarding food consumption.
The orbitofrontal cortex impacts appetite by using sensory and limbic cues to help us. The orbitofrontal cortex is responsible for controlling impulses, valuing rewards, and decision-making. How an individual thinks will affect the things they consume and have an effect on their appetite. It uses sensory cues by sensing the modalities of a food like taste and smell. This sensation shapes our perception of how a food will taste like and make them feel.
Glucose-sensing vagal and sympathetic afferents arise from the liver and gastrointestinal tract; these convey information to the nucleus of the solitary tract (NTS). Unlike their sympathetic sensory counter-parts arising near the portal vein, vagal glucose sensors probably do not contribute to the counter-regulatory response. However, carotid body glucose sensors may contribute to the counter-regulatory response. Information is relayed from NTS to the dorsal motor nucleus of the vagus (DMV) which provides parasympathetic drive to the pancreatic islets and via the parabrachial nucleus (PBN) top supramedullary structures.
Texture affects appetite on the basis of complexity. Now what exactly does complexity mean in regards to food texture? A food with a complex texture, like a salad with berries and nuts, will create more neural responses while chewing (it often also takes longer to chew as well), so your brain feels like it has eaten more, leaving you feeling full after eating less. On the other side of the coin, it is easier to eat more food with little texture. For example, a smoothie with the same amount of spinach, berries, and nuts as the salad from before would be easier to finish due to its lower textural complexity.
Blood sugar inhibits responses in the nucleus of the solitary tract. When there’s too much blood sugar in the body, it inhibits responses in the nucleus of the solitary tract. One way it does so is by appetite regulation. When someone consumes food, it increases their blood sugar levels and when they have high levels, the nucleus of the solitary tract inhibits their appetite. This inhibition controls food intake and prevents people from overconsuming.
CC LICENSED CONTENT, SHARED PREVIOUSLY
“The Use of Functional MRI to Study Appetite Control in the CNS,” Akila De Silva et al.
Provided by: Journal of Diabetes Research
URL: https://www.hindawi.com/journals/jdr/2012/764017/#copyright
License of article: CC BY
“Neural pathways that control the glucose counterregulatory response,” Anthony J. M. Verberne et. al
Provided by: Frontiers in Neuroscience
URL: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3935387/
License of article: CC BY
Reference
Larsen DS, Tang J, Ferguson LR, James BJ. Increased textural complexity in food enhances satiation. Appetite. 2016;105:189-194. doi:10.1016/j.appet.2016.05.029
Seabrook, L. T., & Borgland, S. L. (2020). The orbitofrontal cortex, food intake and obesity.
Journal of psychiatry & neuroscience : JPN, 45(5), 304–312. https://doi.org/10.1503/jpn.190163
Woods, S. C., Lutz, T. A., Geary, N., & Langhans, W. (2006). Pancreatic signals controlling food intake:
insulin, glucagon and amylin. Philosophical transactions of the Royal Society of London. Series
B, Biological sciences, 361(1471), 1219–1235. https://doi.org/10.1098/rstb.2006.1858

