Module 6: Blood Smear Technique and Reticulocyte Counting
Module 6.4: Reticulocyte Procedure
Reticulocyte smear
In the laboratory, you will be preparing a reticulocyte smear from patient blood for practice. This patient is likely not anemic so you will be also provided a pre-made canine reticulocyte smear for practicing reticulocyte enumeration. You are welcome to try to enumerate reticulocyte from the provided blood but it may not be the most rewarding experience.
Supplies needed:
Brightfield Microscope with a 100x objective lens
Specimen: Whole (K3) EDTA blood
Procedure
- Mix 3 drops of New Methylene Blue with 2 drops of well-mixed whole (EDTA) blood
- Incubate the mixture at room temperature for 15 minutes
- Resuspend mixture by mixing gently, but thoroughly
- Make 3 blood smears
- Allow to air dry
Counting reticulocytes
After your sample has air dried you will perform a manual reticulocyte counting using the 100x objective.
- The first step for either a cat or dog is to identify a region of the blood film preparation where the RBCs are evenly distributed.
- On a piece of paper, make two columns. Label one aggregate reticulocytes and the other RBCs. (See the example below. Each box represents a 1000x magnification field on your scope. )
- Canine- Find an area on the blood smear regions of the slide that the RBCs are evenly distributed. Count the total aggregate reticulocyte per 1000 RBCs. Dogs only release aggregated reticulocytes so count all cells with stippling.
- Feline- In cats, the erythrocyte maturation process is a little different than dogs. Aggregate reticulocytes mature into punctate reticulocytes within 12-24 hours; punctate reticulocytes circulate for at least several days (7-10 days) before all the RNA is lost. The reticulocyte count in cat blood should only include the percentage of aggregate reticulocytes; punctate reticulocytes are not included in the standard reticulocyte count because they do not reflect the most recent bone marrow response (e.g. an anemic cat with only punctate reticulocytes is not actively regenerating at this time, but has shown some bone marrow regeneration in the past 7-10 days). Count only the total number of aggregate reticulocytes per 1000 RBCs.
- Below is an image of a feline blood film. Count all the RBC in the field (including aggregate reticulocytes), then count all the reticulocytes. In this image, there are ~42 total RBC (not counting those along the edges that are cut off) and 2 aggregated reticulocytes
Table 6.1: Counting Aggregate Reticulocytes Aggregate reticulocytes
Total RBCs
2 42 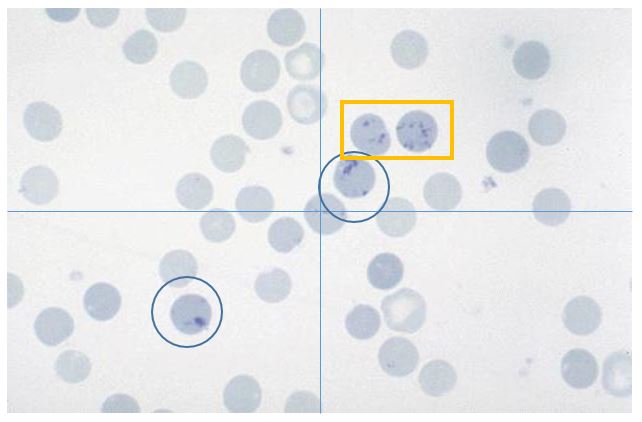
Feline blood film Yellow square = Punctate; Blue circle =Aggregate
Reference interval: Feline aggregates: < 60,000 retic/ uL Canine aggregates: <80,000 retic/uL
- You will continue moving randomly to 100x fields (even if they do not have reticulocytes) until you have reached 1000 RBCs. See the example below.
Aggregate reticulocytes |
Total RBCs |
|---|---|
| 2 | 42 |
| 5 | 54 |
| 9 | 45 |
| 0 | 50 |
| 1 | 49 |
| 3 | 59 |
| 0 | 48 |
| 2 | 52 |
| 1 | 45 |
| 4 | 52 |
| 0 | 42 |
| 1 | 61 |
| 2 | 36 |
| 5 | 62 |
| 6 | 60 |
| 7 | 44 |
| 1 | 51 |
| 0 | 52 |
| 3 | 45 |
| 2 | 47 |
Total |
Total |
| 54 | 996 |
Calculation
In this feline patient, you have counted 54 aggregated reticulocytes per 1000 cells. The next step is to turn this into a percentage, also known as the reticulocyte %.
Reticulocyte % Equation
![]()
Case Example Calculation
![]()
How do I interpret this?
Whenever we generate a percentage, you need to ask yourself. What is this a percentage of? For example, 5% reticulocytes in an animal with a PCV or HCT of 60% is different than an animal with a PCV of HCT of 12%.
There are several ways we can determine a value for the total number of erythrocytes. Generally, we use the absolute reticulocyte count equation, but in instances when we only have a PCV, we will use the corrected reticulocyte equation. Both equations account for the severity of anemia, but the absolute is considered more accurate. For a more thorough description of the two calculations, please see the eClinPath website.
Absolute Reticulocyte Count Equation
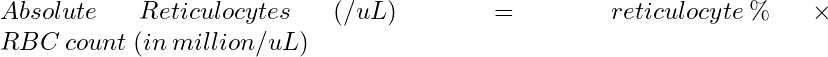
Case Example Calculation
In our patient, we gathered the following data from the CBC.
- HCT: 15%
- RBC count: 1.5 million RBCs
![]()
![]()
So what does this mean?
This value can help us determine the degree of regeneration to better understand the patient’s regenerative response to anemia. We have species-specific reference intervals for these values.
Degree of regeneration |
Feline Absolue aggregate reticulocytes (/uL) |
Canine Absolue aggregate reticulocytes (/uL |
|---|---|---|
| None | < 60,000 | <
<95,000 |
| Mild | 80,000 | 100,000 |
| Moderate | 100,000 | 300,000 |
| Severe | ≥
≥200,000 |
≥
≥500,00 |
Based on the table using the eClinPath website values (these values may vary by geographic region and are considered more of a guideline), our cat has approximately 81,300 reticulocytes per uL, so this cat has a mild regenerative response. How we interpret if this mild response is appropriate or expected will depend on the clinical signs and duration of anemia. This will be discussed more in your medicine and clinical pathology courses.
Knowledge check
Key Takeaways for Evaluating Regeneration
- Reticulocytes are immature RBCs that are the stage just before a mature RBC and after a metarubricyte (nRBC)
- We can use supervital stains, such as new methylene blue to stain the retained ribosomal RNA for reticulocyte enumeration
- We use the term “polychromatophil” when we observe immature RBCs when using Wright Giemsa or DiffQuik. We use the term “reticulocyte” when we use new methylene blue to view and evaluate RBCs.
- We only count aggregate reticulocytes in cats and both punctate and aggregate in dogs
- Large animals tend to not have reticulocytes in circulation, even when very anemic

