Module 9: Urine Culture and Sensitivity
Module 9.5: Determining Antibiotic Sensitivity or Susceptibility After Isolation of the Pathogen
Antibiotic sensitivity testing
Depending on the clinical signs and ISCAID guidelines, you may opt to perform sensitivity (or susceptibility) testing on the bacterial isolate(s) to guide antimicrobial therapy for your patient. The antibiotic sensitivity testing procedure that you will be performing in the laboratory is the same across all testing scenarios whether it be fluid from a septic joint, milk in a cow that has mastitis, or a transtracheal wash from an animal with respiratory disease.
After isolation of the pathogen, there are 4 major steps for the determination of susceptibility patterns for a specific pathogen.
- Using McFarland Standards to semi quantify the number of bacteria plated onto the Mueller-Hinton Antibiotic Sensitivity Plate
- Inoculating the Mueller-Hinton plate with the bacterial suspension
- Addition of the antibiotic discs (Kirby Bauer Disc Diffusion) to determine the zone of inhibition
- Using the Mueller-Hinton Plate results to determine the minimum inhibitory concentration (MIC) of the drugs that the pathogen is sensitivity to using an E-test
Let’s discuss these 4 steps in greater detail.
1. McFarland Standards
The McFarland Standards are used as a reference to adjust the bacterial concentration in a suspension using turbidity to standardize microbial testing and data. If a suspension used is too heavy or too dilute, an erroneous result (either falsely resistant or falsely susceptible) for any given antimicrobial agent could occur.

Knowledge check
2. Antibiotic sensitivity testing
Once the bacterial suspension is prepared and standardized it to 0.5 using the McFarland Standards, this suspension will need to be cultured WITH the specific antibiotics you are looking to use to target the infection in your patients. Generally, these antibiotics are selected by the reference laboratory and based on the original sample type.
Preparing an antibiotic sensitivity plate
In this laboratory, your antibiotic plates, using the Mueller-Hinton plate, have been prepared for you and stamped with several discs that are impregnated with various antibiotics (Kirby Bauer Disc Diffusion Test)
The following steps were taken to prepare your antibiotic sensitivity plate.
- The Mueller-Hinton plate was inoculated by swabbing in three directions to evenly (and thin) coat the entire plate with the suspension. The plate was turned 45 degrees each time it was streaked. There should not be any areas on the plate that have not been entirely inoculated with bacterial broth.

- Antibiotic discs were stamped (using a stamping device) and incubated at 37 degrees F overnight.
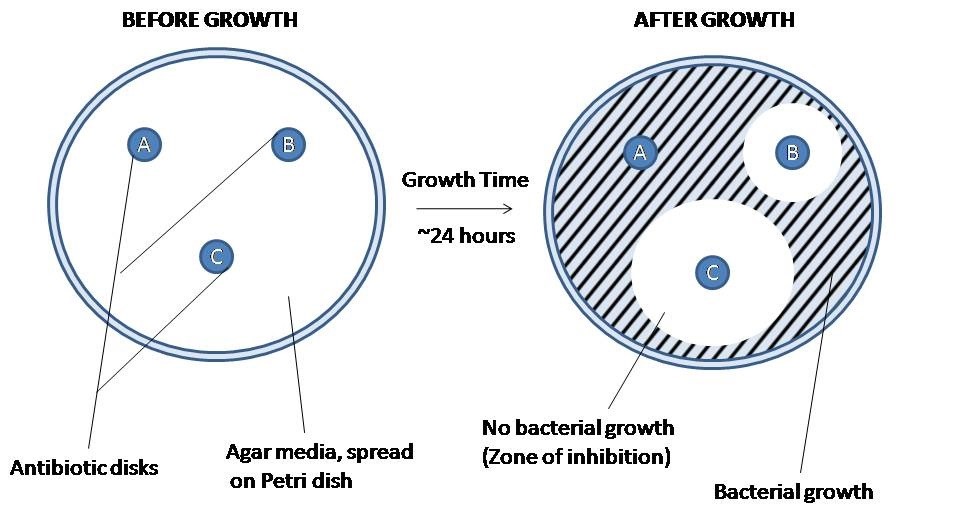
3. Interpreting your Kirby Bauer Disc Diffusion
After the isolate has been allowed to grow overnight, you should be able to observe the zone of inhibition (ZOI) surrounding the antibiotic discs.
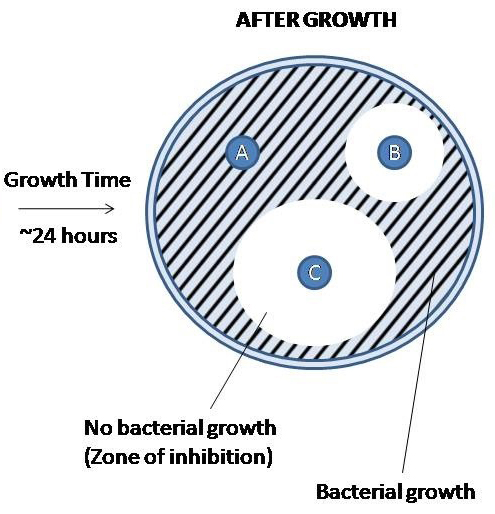
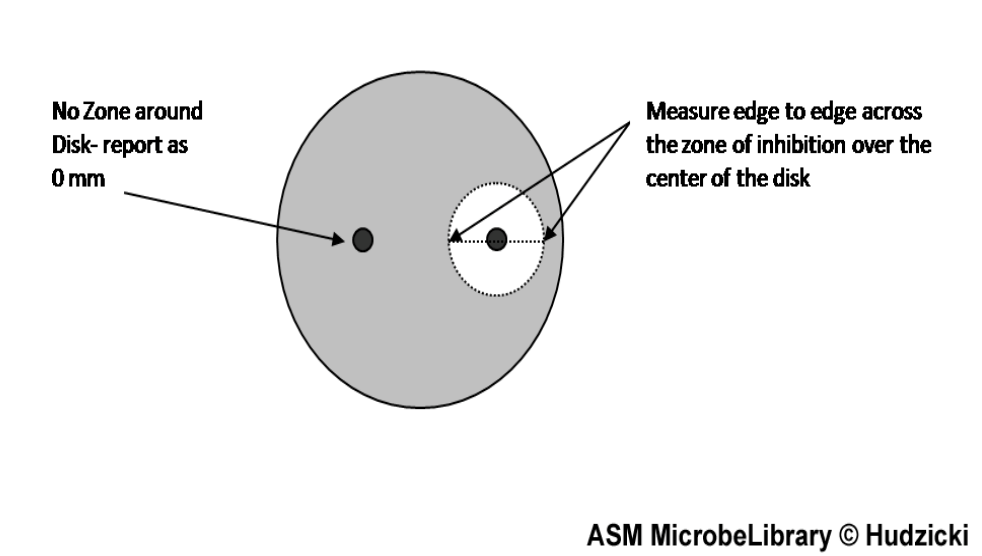
These zones are regions of the plate in which the antibiotic disc was able to diffuse across the agar and inhibit bacterial growth. The diameter of the zones is measured (millimeters) to determine the size of the zone of inhibition. The size of a normal zone of inhibition varies from drug to drug based on the properties of the drugs (predominately molecule size) as well as bacterial isolate. Larger molecules will not diffuse as far as smaller molecules. Based on the measurement of the ZOI, the drug is categorized as resistant, intermediate, and sensitive. It is important to remember that drugs being resistant, sensitive, or intermediate are based on behavior in an in vitro setting using a pure isolate and may not be predictive of biological behavior. This data is only used to help guide your decision based on the clinical presentation.
The table below is an example of the reference intervals for several drugs (bolded) and bacteria (white row) with each ZOI.
Size of Zone of Inhibition (ZOI in mm) |
||||
|---|---|---|---|---|
Antibiotic: |
Disc |
Resistant (R) |
Intermediate (I) |
Sensitive (S) |
Sulfamethoxazole-Trimethoprim |
SXT | |||
| Staphylococcus spp. | ≤10 | 11-15 | ≥16 | |
| Enterobacteriaceae | ≤10 | 11-15 | ≥16 | |
| Haemophilus influenzae | ≤10 | 11-15 | ≥16 | |
| Streptococcus pneumoniae | ≤15 | 16-19 | ≥19 | |
Amoxicillin/Clavulanic acid |
AMC-30 | |||
| Staphylococcus spp. | ≤19 | ≥20 | ||
| Other organisms | ≤13 | 14-17 | ≥18 | |
Ciprofloxacin |
CIP-5 | |||
| ≤15 | 16-20 | ≥21 | ||
Nitrofurantoin |
F/M-300 | |||
| ≤14 | 15-16 | ≥17 | ||
Ampicillin |
AM-10 | |||
| Staphylococcus sp. | ≤28 | ≥29 | ||
| Enterobacteriaceae | ≤13 | 14-16 | ≥17 | |
| Streptococcus (not S. pneumoniae) | ≤18 | 19-25 | ≥26 | |
Ceftriaxone |
CRO-30 | |||
| ≤13 | 14-20 | ≥21 | ||
Knowledge check
4. Determining the minimum inhibitory concentration (MIC)
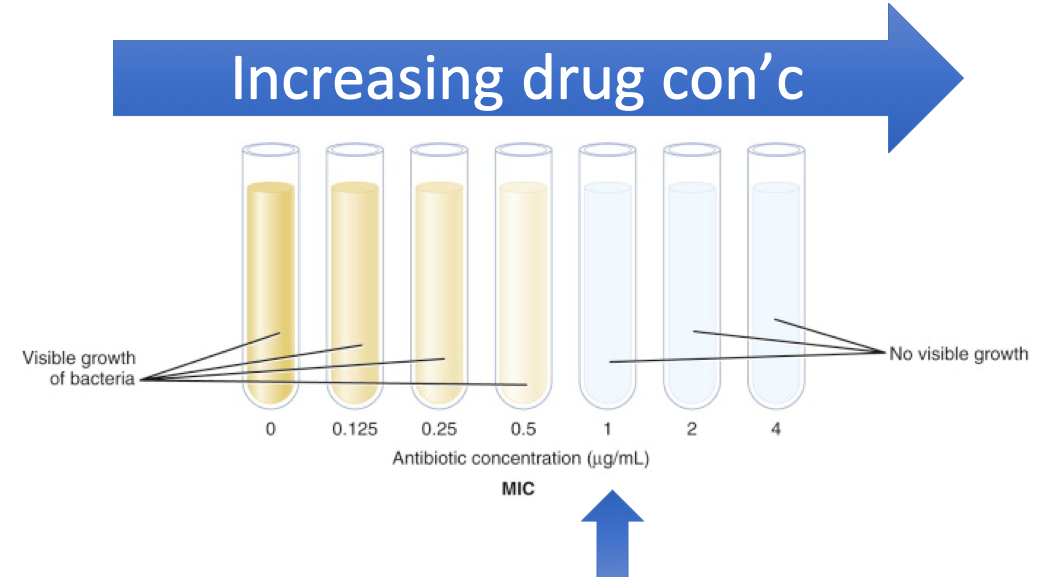
The minimum inhibitory concentration (MIC) is the lowest concentration of a chemical, usually a drug, which inhibits visible bacterial in an in vitro setting. This is the bacteriostatic effect of the drug. If we wanted to determine the bactericidal effect of the drug we would need to measure the minimum bactericidal concentration (MBC). This will not be performed in the lab.
For the image below (from Dr. Brown’s lecture notes) you see that the tubes are arranged in increasing antibiotic concentration. Once the antibiotic concentration reaches 1 ug/mL the visible microbial growth can no longer be detected. Thus, the MIC for this isolate is 1 ug/mL.
In addition to using serial dilutions in test tubes, test strips that are impregnated with increasing concentration of a single drug called “E-test” can be applied directly to a Mueller-Hinton plate. The strip is ticked with various concentrations of the drug. The MIC is determined by reading where the edge of the inhibition ellipse intersects with the side of the E-test strip. In the example below the MIC is 0.125 ug/mL for Amoxicillin (beta-lactam antibiotic).
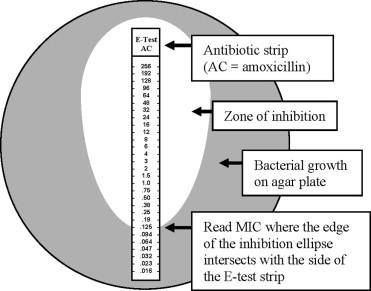
Since E-tests are expensive, the Kirby Bauer Disc Diffusion test is performed first to evaluate which drugs a bacterial isolate is sensitive to before selecting which drugs to determine the MIC on. Due to time constraints, you will be applying an E-test directly to your Kirby Bauer test in the lab to determine the MIC of a certain drug (to be determined based on your isolates). The interpretation of specific cut-points for S, I, and R are determined by CSLI guidelines and vary for each drug and bacterial isolate.

